Beautiful Plants For Your Interior
Summary
Roparil, also recognized as (–)-2β-Carbomethoxy-3β-phenyltropane, WIN 35,065-2, or β-CPT, is a stimulant compound primarily employed in scientific research. Derived from methylecgonidine, troparil belongs to the phenyltropane class of drugs and acts as a potent dopamine reuptake inhibitor (DRI). Compared to cocaine, trail exhibits several times greater potency in inhibiting dopamine reuptake, but it is less effective as a serotonin reuptake inhibitor.
One noteworthy distinction of troparil is its extended duration of action, which is attributed to its unique chemical structure. Unlike cocaine, the trail features a non-hydrolyzable carbon-carbon bond directly connecting the phenyl ring to the tropane ring. This structural alteration eliminates the local anaesthetic properties typically associated with cocaine, rendering troparil a pure stimulant.
Moreover, this structural modification contributes to troparil’s slightly reduced cardiotoxicity when compared to cocaine, making it a potentially safer option. Troparil is commonly available in various forms, including tartrate salt, hydrochloride salt, naphthalene disulfonate salt, and the free base. These different formulations cater to specific research needs within the scientific community.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 74163-84-1 |
|---|---|
| PubChem CID | 170832 |
| ChemSpider | 149355 |
| UNII | 11M96D7J17 |
| ChEMBL | ChEMBL298684 |
| CompTox Dashboard (EPA) | DTXSID30964641 |
| Chemical and physical data | |
| Formula | C16H21NO2 |
| Molar mass | 259.349 g·mol−1 |
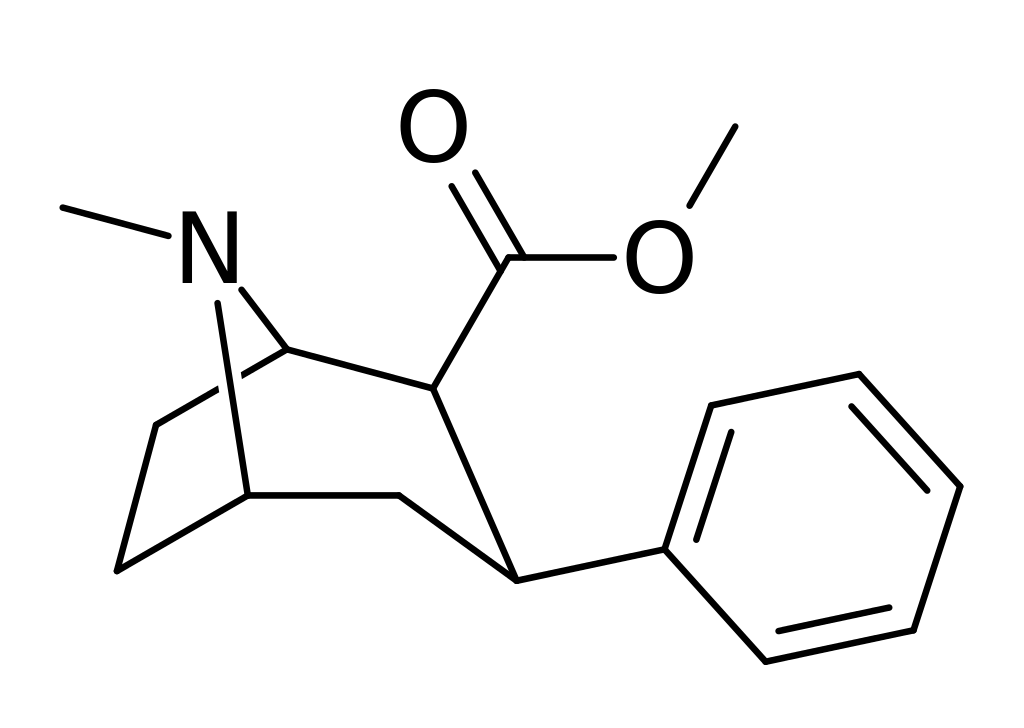
Background
The initial documented synthesis of troparil and its counterpart, WIN 35428, was carried out by Clarke and colleagues in the 1970s. Evidently, their objective was to disentangle the stimulant effects of cocaine from its associated toxicity and addictive properties. Notably, among the phenyltropane compounds, troparil stands out as the sole one with a higher affinity for the norepinephrine transporter (NET) compared to its affinity for the dopamine transporter (DAT). This unique attribute sets it apart in the realm of phenyltropanes.
Application
Phenyltropanes are generally believed to possess a lower risk of abuse and dependency when compared to cocaine.
Troparil plays a crucial role in scientific research focused on the dopamine reuptake transporter. Radiolabelled versions of troparil, such as 3H-radiolabelled forms, have been employed in both human and animal studies to map the distribution of dopamine transporters within the brain. Additionally, it serves as a valuable tool in animal research exploring stimulant drugs, offering an alternative to cocaine with similar effects. This approach circumvents the stringent licensing requirements associated with the use of cocaine itself.
In animal studies, troparil has demonstrated effects akin to those of cocaine. However, recreational use of this compound remains exceptionally rare. Despite its relative ease of synthesis through the reaction of methylecgonidine with phenylmagnesium bromide, the limited availability of methylecgonidine and the demanding conditions required for the synthesis render the production of this compound beyond the capabilities of most illicit drug manufacturers. Consequently, legitimate sources of the trail are only accessible in minuscule quantities at a considerable cost.
Legality
The legal classification of troparil remains ambiguous, with some considerations pointing towards its potential categorization as a controlled substance analogue of cocaine in the United States due to its structural similarity. In Canada, the legal status of trail, along with numerous other cocaine analogues, hinges on whether the compound can be traced back to ecgonine, coca, or cocaine derivatives, as stipulated in the language pertaining to the inclusion of coca in Schedule 1 of the Controlled Drugs and Substances Act.
FAQ
1. What is Troparil? Troparil is a chemical compound that belongs to the class of substances known as phenyltropanes. It is primarily used for its stimulant properties and has been investigated for its potential use in research and pharmacology.
2. What are the main uses of Troparil? Troparil is not approved for medical or recreational use. It has been studied primarily for its potential as a stimulant, but its use is limited to scientific research and has no established medical applications.
3. Is Troparil legal? The legal status of Troparil varies by country and region. It is generally considered a controlled substance in many places due to its potential for misuse and abuse. Before obtaining or using Troparil, it is crucial to research and understand the specific regulations and laws in your area.
4. What are the potential risks and side effects of Troparil? Troparil is a stimulant, and like other stimulants, it can have various side effects, including increased heart rate, elevated blood pressure, anxiety, insomnia, and the potential for addiction. Its long-term effects and risks are not well-documented due to limited research.
5. Can Troparil be used as a replacement for other stimulants like cocaine or amphetamines? Troparil has a similar chemical structure to cocaine, and it has been explored as a potential substitute in research settings. However, it is essential to note that Troparil may carry similar risks and dangers as other stimulants, and its use is not a safer alternative.
6. How is Troparil typically administered? Troparil is typically administered through oral ingestion or intravenous injection. The specific method of administration may vary depending on the research or experimentation being conducted.
7. Is Troparil used in medical treatments or therapies? As of my last knowledge update in September 2021, Troparil had no approved medical applications. It was primarily used in scientific research to understand its pharmacological properties better.
8. Can I purchase Troparil for personal use? In most regions, Troparil is a controlled substance, and it is illegal to purchase or possess it without proper authorization. Attempting to obtain Troparil without a valid research or regulatory reason may result in legal consequences.
9. Is Troparil safe to use recreationally or for self-experimentation? Troparil has not been thoroughly studied for recreational use, and its safety profile is not well-established. Engaging in recreational use or self-experimentation with Troparil is strongly discouraged due to potential health risks.
10. Where can I find more information about Troparil? For scientific and research-related information, you can refer to peer-reviewed journals academic publications, or consult with professionals in the field of pharmacology and chemistry. Always ensure that you are following the legal and ethical guidelines in your jurisdiction when seeking information or engaging in research involving Troparil.
References
- Runyon SP, Carroll FI (2006). “Dopamine transporter ligands: recent developments and therapeutic potential”: This publication discusses the advancements in dopamine transporter ligands, including Troparil. It explores the therapeutic potential of these compounds, shedding light on their pharmacological properties and potential applications in the field of medicine.
- Carroll FI, Kotian P, Dehghani A, Gray JL, Kuzemko MA, Parham KA, et al. (January 1995). “Cocaine and 3 beta-(4′-substituted phenyl)tropane-2 beta-carboxylic acid ester and amide analogues”: This study delves into the development of analogues of cocaine, including Troparil. It highlights their high affinity and selectivity for the dopamine transporter, which plays a critical role in understanding the pharmacological effects of these compounds.
- Phillips K, Luk A, Soor GS, Abraham JR, Leong S, Butany J (2009): This review focuses on the cardiotoxic effects of cocaine, including its impact on the cardiovascular system. While not specifically about Troparil, it provides valuable insights into the pathophysiology, pathology, and treatment options associated with stimulants like Troparil.
- U.S. Patent 3,813,404: This patent likely pertains to a specific aspect of Troparil or its synthesis. Patents often contain detailed information about the compound’s invention, application, or manufacturing processes.
- Clarke RL, Daum SJ, Gambino AJ, Aceto MD, Pearl J, Levitt M, et al. (November 1973): This research explores compounds affecting the central nervous system, including certain phenyltropane derivatives. While not directly about Troparil, it provides foundational knowledge related to the chemical family to which Troparil belongs.
- Wee S, Carroll FI, Woolverton WL (February 2006): This study investigates the dopamine transporter binding rates in vivo and their association with the reinforcing efficacy of stimulants. While not Troparil-specific, it sheds light on the mechanisms underlying the effects of stimulants.
- Kimmel HL, O’Connor JA, Carroll FI, Howell LL (January 2007): This research explores the onset and selectivity of dopamine transporter binding and how these factors predict the stimulant and reinforcing effects of cocaine analogs, which can be relevant for understanding Troparil’s effects.
- Ritz MC, Boja JW, Grigoriadis D, Zaczek R, Carroll FI, Lewis AH, Kuhar MJ (November 1990): This study investigates ligands for cocaine receptors in the striatum, which contributes to the understanding of the neuropharmacology of compounds like Troparil.
- Scheffel U, Boja JW, Kuhar MJ (1989): This research discusses the labeling of cocaine receptors in vivo with various ligands, providing insights into the binding characteristics of these compounds in the brain.
- Zakusov VV, Naumova BI (1985): This publication, likely in Russian, discusses the pharmacology of Troparil or related compounds, providing information on its effects and properties.
- Balster RL, Carroll FI, Graham JH, Mansbach RS, Rahman MA, Philip A, et al. (December 1991): This study explores the discriminative stimulus effects of potent substituted-3 beta-phenyltropane analogs of cocaine, shedding light on their similarities to cocaine.
- Xu L, Kelkar SV, Lomenzo SA, Izenwasser S, Katz JL, Kline RH, Trudell ML (March 1997): This research involves the synthesis and evaluation of 2-substituted 3 beta-phenyltropane derivatives, which can provide insights into the structure-activity relationships of compounds related to Troparil.
- Kline RH, Wright J, Fox KM, Eldefrawi ME (July 1990): This research pertains to the synthesis of 3-arylecgonine analogues as inhibitors of cocaine binding and dopamine uptake, contributing to our understanding of compounds related to Troparil.
- Xu L, Trudell ML (1996): This publication discusses the stereoselective synthesis of 2β-carbomethoxy-3β-phenyltropane derivatives, which can be relevant to the synthesis and characterization of Troparil and related compounds.
- Milius RA, Saha JK, Madras BK, Neumeyer JL (May 1991): This research explores the synthesis and receptor binding of N-substituted tropane derivatives, including compounds with high affinity for the cocaine receptor.
- “Controlled Drugs and Substances Act (S.C. 1996, c. 19)”: This is a reference to the Canadian legislation governing controlled substances, which includes regulations related to substances like Troparil. It’s important to consult such legal documents to understand the regulatory framework surrounding Troparil in Canada.