- 1 Summary
- 2 FAQ
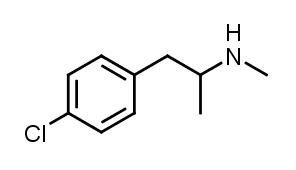
- 2.1 1. What is para-chloromethamphetamine (4-CMA)?
- 2.2 2. What are the effects of 4-CMA on serotonin levels?
- 2.3 3. Are there any long-term effects associated with 4-CMA use?
- 2.4 4. How does 4-CMA compare to methamphetamine regarding inducing conditioned taste aversion?
- 2.5 5. What distinguishes 4-CMA from amphetamine and methamphetamine compounds?
- 2.6 6. Where was 4-CMA first identified outside of laboratory settings?
- 2.7 7. Is 4-CMA a legal substance?
- 3 References
Summary
Para-chloromethamphetamine, also called 4-chloromethamphetamine and 4-CMA, is a stimulant that serves as the N-methyl derivative and prodrug of the neurotoxic compound para-chloroamphetamine (4-CA). This substance has been observed to decrease serotonin levels in rats. Further exploration into the enduring effects of chloroamphetamines revealed that the administration of 4-CMA led to a prolonged reduction in serotonin levels and the activity of tryptophan hydroxylase in the brain one month after a single dose of the drug was administered.
In studies involving rats, it was found that 4-chloromethamphetamine was more effective at inducing conditioned taste aversion compared to methamphetamine.
During the 1960s, 4-chloromethamphetamine was subject to further investigation alongside 4-CA. It was noted that these compounds differed from their parent amphetamine and methamphetamine substances, as they exhibited only a mild central stimulant effect in both animals and humans. Interestingly, they seemed to act more like antidepressants rather than typical stimulants.
It is noteworthy that 4-chloromethamphetamine was identified outside of laboratory settings for the first time at the Tomorrowland festival edition in 2015 when a tablet containing the substance was found in the possession of a drug dealer (see picture).
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 30572-91-9 |
|---|---|
| PubChem CID | 3128 |
| ChemSpider | 3016 |
| UNII | IXP5G5WMYA |
| CompTox Dashboard (EPA) | DTXSID00897230 |
| Chemical and physical data | |
| Formula | C10H14ClN |
| Molar mass | 183.68 g·mol−1 |
FAQ
1. What is para-chloromethamphetamine (4-CMA)?
Para-chloromethamphetamine, also known as 4-CMA, is a stimulant drug that acts as the N-methyl derivative and prodrug of another neurotoxic drug called para-chloroamphetamine (4-CA).
2. What are the effects of 4-CMA on serotonin levels?
4-CMA has been found to decrease serotonin levels in laboratory studies involving rats. This effect can have implications for mood and neurotransmitter function.
3. Are there any long-term effects associated with 4-CMA use?
Research has shown that the administration of 4-CMA can lead to a prolonged reduction in serotonin levels and the activity of tryptophan hydroxylase in the brain, even one month after a single drug dose is administered.
4. How does 4-CMA compare to methamphetamine regarding inducing conditioned taste aversion?
Studies on rats have indicated that 4-chloromethamphetamine is more potent inducing conditioned taste aversion than methamphetamine. This suggests that it may have distinct effects on behavior and aversion.
5. What distinguishes 4-CMA from amphetamine and methamphetamine compounds?
In investigations during the 1960s, 4-chloromethamphetamine was noted for its differences from amphetamine and methamphetamine substances. It exhibited only a mild central stimulant effect in both animals and humans and appeared to act more like an antidepressant rather than a typical stimulant.
6. Where was 4-CMA first identified outside of laboratory settings?
4-Chloromethamphetamine was discovered outside of laboratory settings for the first time at the Tomorrowland festival edition in 2015 when a tablet containing the substance was found in the possession of a drug dealer.
7. Is 4-CMA a legal substance?
The legal status of 4-CMA may vary by country and region. Knowing local regulations and laws regarding its possession and use is essential. Always prioritize safety and legal compliance when dealing with such substances.
References
- Johnson MP, Conarty PF, Nichols DE (July 1991). “Neurochemical Properties of MDMA Analogues”. European Journal of Pharmacology. 200 (1): 9–16. doi:10.1016/0014-2999(91)90659-E. PMID 1685125.
- Fuller RW, Baker JC, Perry KW, Molloy BB (October 1975). “Comparing the Effects of Amphetamine Derivatives in Rats”. Neuropharmacology. 14 (10): 739–746. doi:10.1016/0028-3908(75)90099-4. PMID 1196472. S2CID 9620299.
- Murnane KS, Perrine SA, Finton BJ, Galloway MP, Howell LL, Fantegrossi WE (April 2012). “Amphetamine Derivatives and Their Effects on Behavior and Neurochemistry in Mice”. Psychopharmacology. 220 (3): 495–508. doi:10.1007/s00213-011-2504-0. PMC 3289749. PMID 21993877.
- Pletscher A, Burkard WP, Bruderer H, Gey KF (November 1963). “Reducing Cerebral 5-Hydroxytryptamine Levels with an Arylalkylamine”. Life Sciences. 2 (11): 828–833. doi:10.1016/0024-3205(63)90094-8. PMID 14078137.
- Fuller RW, Hines CW, Mills J (April 1965). “Lowering Brain Serotonin Levels with Chloramphetamines”. Biochemical Pharmacology. 14 (4): 483–488. doi:10.1016/0006-2952(65)90221-2. PMID 14322972.
- Sanders-Bush E, Bushing JA, Sulser F (January 1975). “Long-term Effects of Amphetamine Derivatives on Serotonergic Mechanisms”. The Journal of Pharmacology and Experimental Therapeutics. 192 (1): 33–41. PMID 1123726.
- Booth DA, Pilcher CW, D’Mello GD, Stolerman IP (December 1977). “Comparative Potencies of Stimulants in Taste Aversion Experiments”. British Journal of Pharmacology. 61 (4): 669–677. doi:10.1111/j.1476-5381.1977.tb07560.x. PMC 1668069. PMID 597669.
- van Praag HM, Korf J, van Woudenberg F, Kits TP (July 1968). “Influencing Indoleamine Metabolism with an Antidepressant Amphetamine Derivative”. Psychopharmacologia. 13 (2): 145–160. doi:10.1007/BF00404812. PMID 5678577. S2CID 30028917.
- Kits TP, van Praag HM (1970). “Antidepressant Effects of p-Chloro-N-Methylamphetamine”. Acta Psychiatrica Scandinavica. 46 (4): 365–373. doi:10.1111/j.1600-0447.1970.tb02126.x. PMID 5502782. S2CID 2211532.
- van Praag HM, Korf J, van Woudenberg F (December 1970). “Influence of Chlorinated Amphetamine Derivatives on Serotonin Synthesis in Humans”. Psychopharmacologia. 18 (4): 412–420. doi:10.1007/BF00402767. PMID 4923523. S2CID 27509683.
- van Praag HM, Schut T, Bosma E, van den Bergh R (March 1971). “Therapeutic Effects of Chlorinated Amphetamine Derivatives in Depressive Patients”. Psychopharmacologia. 20 (1): 66–76. doi:10.1007/BF00404060. PMID 5565748.
- Blanckaert P, Vanquekelberghe S, Coopman V, Risseeuw MD, Van Calenbergh S, Cordonnier J (July 2018). “Identification of 4-Chloromethamphetamine (4-CMA) in Seized Ecstasy and Its Impact on Public Health”. Forensic Science International. 288: 173–180. doi:10.1016/j.forsciint.2018.04.023. hdl:1854/LU-8569680. PMID 29753935. S2CID 21720366.

Leave a Reply