Beautiful Plants For Your Interior
Summary
Diazepam (also referred to as chlorodiazepam and 2′-chloro-diazepam) is a benzodiazepine compound and serves as a functional analogue of diazepam. This chemical entity was initially synthesized by Leo Sternbach and his research team at Hoffman-La Roche in 1960. Despite its origins, diazepam has not obtained approval for clinical use as a medication and is available in the market as an unscheduled substance. To date, its efficacy and safety in humans remain untested.
In preclinical animal models, diazepam exhibits effects akin to those of diazepam. These effects encompass prolonged anxiolytic (anxiety-reducing), anticonvulsant (seizure-inhibiting), hypnotic (sleep-inducing), sedative (calming), skeletal muscle relaxant, and amnestic (memory-impairing) properties.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 2894-68-0 |
|---|---|
| PubChem CID | 76168 |
| ChemSpider | 68652 |
| UNII | 070818R7PB |
| CompTox Dashboard (EPA) | DTXSID30183138 |
| Chemical and physical data | |
| Formula | C16H12Cl2N2O |
| Molar mass | 319.19 g·mol−1 |
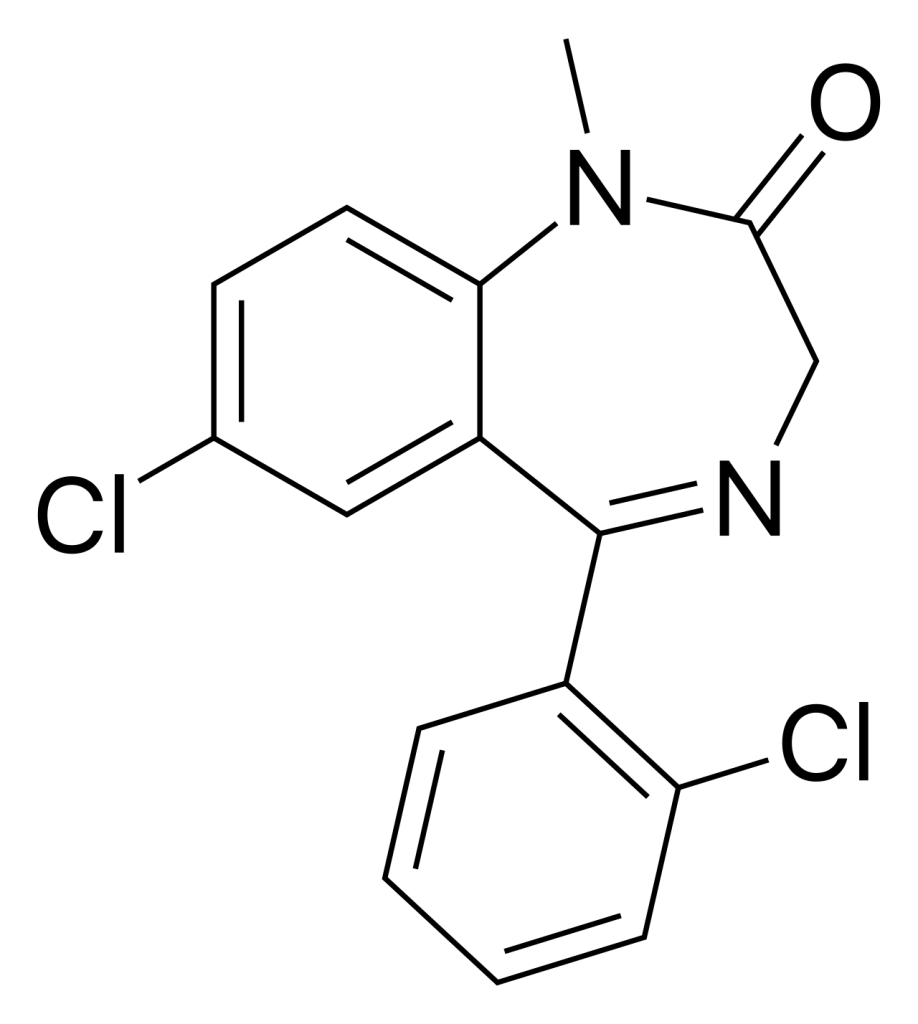
Metabolism
The metabolism of diazepam has been extensively studied, indicating that it possesses an approximate elimination half-life of 42 hours. The compound undergoes N-demethylation, leading to the formation of delorazepam. After the administration of the parent compound, delorazepam can be detected in urine for up to six days. Furthermore, the analysis of metabolites also revealed the presence of lorazepam and lormetazepam, which remain detectable in urine for 19 and 11 days, respectively. This suggests concurrent hydroxylation by cytochrome P450 enzymes alongside the N-demethylation process.
Legal Status:
United Kingdom: In the United Kingdom, diazepam has been categorized as a Class C drug following the May 2017 amendment to The Misuse of Drugs Act 1971. This classification places it in the same category as several other benzodiazepine drugs.
United States: On December 23, 2022, the Drug Enforcement Administration (DEA) initiated consideration of placing diclazepam under temporary Schedule I status. Later, on July 25, 2023, the DEA published a pre-print notice announcing that diclazepam would be temporarily scheduled as a controlled substance under Schedule I from 07/26/2023 to 07/26/2025.
FAQ
1. What is Diclazepam?
Diclazepam, also known as chlorodiazepam and 2′-chloro-diazepam, is a benzodiazepine and a functional analog of diazepam. It was initially synthesized by Leo Sternbach and his team at Hoffman-La Roche in 1960. Although it is not approved for medical use, it is available as an unscheduled substance.
2. What are the effects of Diclazepam?
In animal models, Diclazepam exhibits effects similar to diazepam. These effects include long-acting anxiolytic (anxiety-reducing), anticonvulsant (seizure control), hypnotic (sleep-inducing), sedative (calming), skeletal muscle relaxant, and amnestic (memory-impairing) properties.
3. How is Diclazepam metabolized in the body?
Diclazepam undergoes metabolism in the body. It has an approximate elimination half-life of 42 hours. During metabolism, it is N-demethylated to produce delorazepam, a metabolite that can be detected in urine for up to six days. Other metabolites detected include lorazepam and lormetazepam, which can be detectable in urine for 19 and 11 days, respectively. This indicates that hydroxylation by cytochrome P450 enzymes occurs concurrently with N-demethylation.
4. What is the legal status of Diclazepam?
- United Kingdom: In the UK, Diclazepam has been classified as a Class C drug, along with several other benzodiazepine drugs, under the Misuse of Drugs Act 1971, following an amendment in May 2017.
- United States: The Drug Enforcement Administration (DEA) began considering placing Diclazepam under temporary Schedule I status on December 23, 2022. On July 25, 2023, the DEA published a pre-print notice stating that Diclazepam would become temporarily scheduled as a Schedule I controlled substance from 07/26/2023 to 07/26/2025.
5. Can Diclazepam be used for medical purposes?
Diazepam is not approved for use as a medication, and its efficacy and safety have not been tested in humans. It is primarily sold as an unscheduled substance and has been associated with recreational and non-medical use.
6. Is Diclazepam the same as diazepam?
Diclazepam is a functional analogue of diazepam, but it is a distinct compound. It shares similarities in its effects with diazepam but should not be confused with it. Diazepam is an approved medication with established medical uses, whereas Diclazepam is not approved for any medical purpose.
7. What precautions should be taken with Diclazepam use?
Given its status as an unscheduled substance and the potential risks associated with benzodiazepines, it is essential to exercise caution when considering the use of Diclazepam. It should only be used under the guidance of a qualified healthcare professional if prescribed for a specific medical condition.
8. Is Diclazepam associated with dependence or withdrawal symptoms?
Benzodiazepines, including compounds like Diazepam, have the potential for dependence when used over extended periods. Abrupt discontinuation of benzodiazepines can lead to withdrawal symptoms. It is crucial to use these substances responsibly and as prescribed when used for medical purposes.
References
- Anvisa Resolution: In March 2023, Anvisa (Collegiate Board Resolution No. 784) published an update regarding the control of narcotic, psychotropic, precursor, and other substances in Brazilian Portuguese. This resolution addresses regulatory control in Brazil.
- Characterization and Metabolism of Diclazepam: A study conducted in July-August 2014 by Moosmann, Bisel, and Auwärter delves into the characterization of diclazepam, a designer benzodiazepine, and offers preliminary data regarding its metabolism and pharmacokinetics. This research contributes to our understanding of this compound.
- US Patent: US Patent 3136815 pertains to “Amino substituted benzophenone oximes and derivatives thereof.” While not directly related to diclazepam, patents can sometimes provide insights into the chemical structures and derivatives associated with specific compounds.
- Detectability of Designer Benzodiazepines: A study by Pettersson Bergstrand, Helander, Hansson, and Beck in April 2017 examines the detectability of designer benzodiazepines in various immunochemical screening assays. This research is essential for identifying these substances in drug tests.
- Blood Concentrations of Designer Benzodiazepines: In November 2016, Høiseth, Tuv, and Karinen studied the blood concentrations of new designer benzodiazepines in forensic cases. Such investigations are crucial for understanding the prevalence and impact of these substances in real-world scenarios.
- Experimental vs. Theoretical Values: Manchester, Maskell, and Waters conducted research in March 2018 comparing experimental and theoretical values for log D7.4, pKa, and plasma protein binding of benzodiazepines. This type of analysis helps validate theoretical predictions against experimental data.
- Blood-to-Plasma Ratio and GABAA-Binding Affinity: In July 2022, Manchester, Waters, Haider, and Maskell explored the blood-to-plasma ratio and predicted GABAA-binding affinity of designer benzodiazepines. Understanding the affinity of these compounds for receptors is crucial for evaluating their pharmacological effects.
- Pharmacokinetics of Chlordesmethyldiazepam: Bareggi, Truci, Leva, Zecca, Pirola, and Smirne’s 1988 research focused on the pharmacokinetics and bioavailability of intravenous and oral chlordesmethyldiazepam in humans. While not directly related to diclazepam, this information can be valuable in understanding the broader benzodiazepine class.
- Misuse of Drugs Act 1971 (Amendment): In 2017, the Misuse of Drugs Act 1971 was amended, impacting the classification and control of various substances, including designer benzodiazepines like diclazepam.
- DEA Scheduling: On December 23, 2022, the Drug Enforcement Administration (DEA) initiated consideration to temporarily place diclazepam under Schedule I status. On July 25, 2023, a pre-print notice was published, indicating that diclazepam would become temporarily scheduled as a Schedule I controlled substance from 07/26/2023 to 07/26/2025 in the United States. These actions are part of the regulatory control of this substance.