Beautiful Plants For Your Interior
Summary
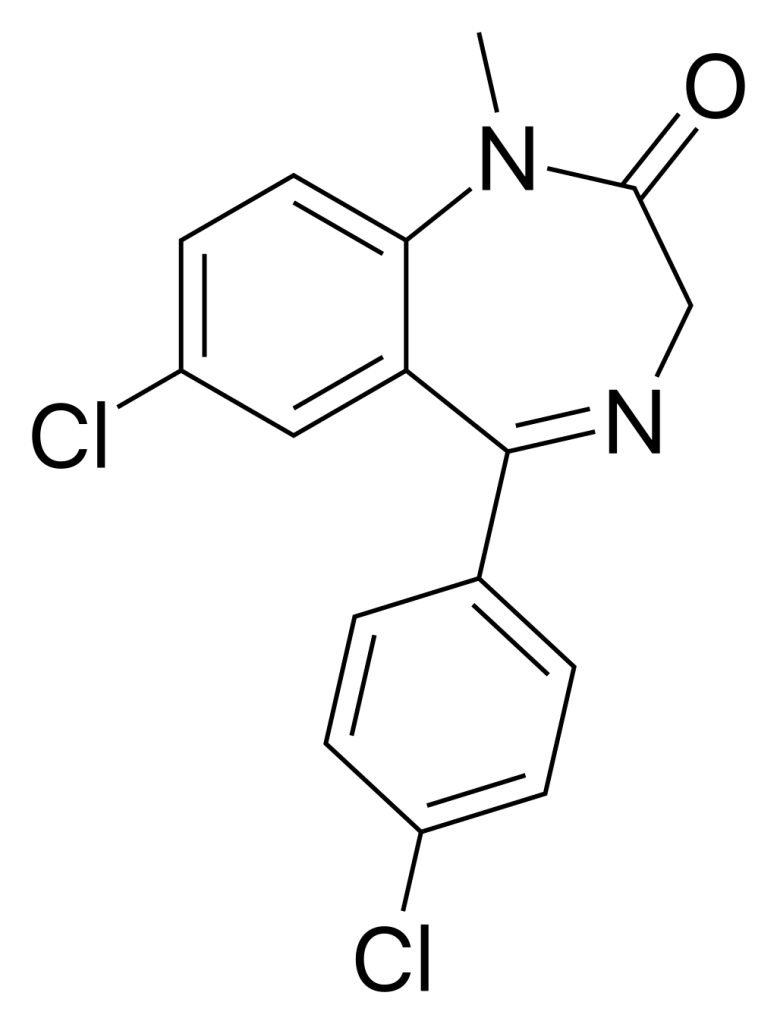
Ro5-4864, also known as 4′-chlorodiazepam, is a benzodiazepine derivative with its origins traced back to diazepam. Unlike the majority of benzodiazepine derivatives, Ro5-4864 exhibits a unique pharmacological profile. It does not display an affinity for GABAA receptors, thus lacking the typical effects associated with benzodiazepines.
Instead, Ro5-4864 manifests as a soothing substance, but it is distinctive in that it can also induce convulsions and anxiogenic responses. This unusual behaviour is attributed to its interaction with the “peripheral benzodiazepine receptor,” which has since been renamed as the mitochondrial translocator protein 18kDa (TSPO).
Interestingly, despite its propensity to induce convulsions, Ro5-4864 has shown promise at lower doses as a neuroprotective agent. As a result, it has gained significant attention in the realm of research, particularly in investigating the role of the TSPO protein in neurotoxicity.
In addition to its neuroprotective properties, in vitro studies and experiments using rodent models have hinted at the potential for Ro5-4864 to have analgesic, antidepressant, cardioprotective, and even anti-cancer effects. This multifaceted nature of Ro5-4864 has led to extensive exploration in various research areas, offering intriguing possibilities for future medical and scientific developments.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 14439-61-3 |
|---|---|
| PubChem CID | 1688 |
| ChemSpider | 1625 |
| UNII | 2QW0IK1742 |
| CompTox Dashboard (EPA) | DTXSID3041116 |
| ECHA InfoCard | 100.162.290 |
| Chemical and physical data | |
| Formula | C16H12Cl2N2O |
| Molar mass | 319.185 g·mol−1 |
FAQ
- What is Ro5-4864?
- Ro5-4864, also known as 4′-chlorodiazepam, is a chemical compound derived from diazepam, belonging to the class of benzodiazepines. It stands out from traditional benzodiazepines due to its unique pharmacological properties.
- How does Ro5-4864 differ from typical benzodiazepines?
- Unlike most benzodiazepine derivatives, Ro5-4864 does not exhibit affinity for GABAA receptors. Consequently, it lacks the typical effects associated with benzodiazepines, such as sedation or anxiety reduction. Instead, Ro5-4864 has sedative qualities but can also provoke convulsions and anxiety.
- What is the “peripheral benzodiazepine receptor” or TSPO that Ro5-4864 interacts with?
- The “peripheral benzodiazepine receptor,” also known as mitochondrial translocator protein 18kDa (TSPO), is a protein located in the mitochondria. Ro5-4864 is a potent ligand for TSPO, and its interaction with this protein is a critical factor in its unique pharmacological effects.
- What are the practical applications of Ro5-4864?
- Ro5-4864 has shown promise as a neuroprotective agent when administered at lower doses. Researchers widely use it to study the role of TSPO in neurotoxicity. It has also been explored for its potential analgesic, antidepressant, cardioprotective, and anti-cancer effects.
- Is Ro5-4864 currently used in medical treatments?
- Ro5-4864 is primarily a research compound and is not commonly used in clinical medical treatments. Its pharmacological profile and potential benefits are still under investigation, and further research is needed to determine its safety and efficacy for specific medical conditions.
- Are there any known side effects or risks associated with Ro5-4864?
- Ro5-4864 can induce convulsions and anxiety, which limits its direct therapeutic use. Its effects can vary depending on the dose and administration method. As with any research compound, safety and potential risks should be carefully considered.
- Where can I find more information about Ro5-4864 research and studies?
- Research papers, scientific journals, and academic institutions are valuable sources for in-depth information on Ro5-4864. Researchers and scientists have published numerous studies on its pharmacology and potential applications.
- Is Ro5-4864 legal and regulated?
- The legal status of Ro5-4864 may vary by country and jurisdiction. It’s essential to check with local regulations and authorities to determine its legal status and any restrictions on its use or distribution.
- What is the future potential of Ro5-4864 in medical research?
- Ro5-4864’s unique pharmacological properties make it an intriguing compound for further studies. As research continues, it may unlock new insights into neuroprotection, mental health, and potential medical applications.
- Can I purchase Ro5-4864 for personal use?
- Ro5-4864 is primarily intended for research purposes and is not readily available for personal use. It is essential to adhere to legal and ethical guidelines when handling research compounds.
References
- In the world of pharmaceuticals, unique identifiers like US 3136815, patent holder Reeder E, and Sternbach LH, brought to life “Amino substituted benzophenone oximes and derivatives thereof” on June 9, 1964. The patent was skillfully assigned to F Hoffmann La Roche AG.
- In March 2018, Manchester KR, Maskell PD, and Waters L delved into the intriguing comparison of “Experimental versus theoretical log D7.4, pKa, and plasma protein binding values for benzodiazepines appearing as new psychoactive substances” in their publication in Drug Testing and Analysis (Volume 10, Issue 8, Pages 1258–1269). This research was noted for its contribution to the field, with a DOI of 10.1002/dta.2387, PMID 29582576, and S2CID 31098917.
- In May 1982, Patel J and Marangos PJ uncovered the “Differential effects of GABA on peripheral and central type benzodiazepine binding sites in the brain.” Their findings, as presented in Neuroscience Letters (Volume 30, Issue 2, Pages 157–160), highlighted the complexity of benzodiazepine binding in the central nervous system. This research was immortalized with a DOI of 10.1016/0304-3940(82)90289-0 and PMID 6287365, as well as S2CID 19728357.
- In 1983, Weissman BA, Cott J, Hommer D, Quirion R, Paul S, and Skolnick P conducted an in-depth exploration of the “Pharmacological, electrophysiological, and neurochemical actions of the convulsant benzodiazepine Ro 5-4864 (4′-chlordiazepam).” Their findings, published in Advances in Biochemical Psychopharmacology (Volume 38, Pages 139–151), contributed significantly to the understanding of this compound. The study was referenced with a PMID 6670623.
- File SE and Lister RG, in their study published in January 1983, unveiled the intriguing discovery that “The anxiogenic action of Ro 5-4864 is reversed by phenytoin” as reported in Neuroscience Letters (Volume 35, Issue 1, Pages 93–96). The research is still relevant today, with a DOI of 10.1016/0304-3940(83)90532-3, and PMID 6682534, and S2CID 11977458.
- In the same year, File SE, Green AR, Nutt DJ, and Vincent ND opened new horizons with their findings on “On the convulsant action of Ro 5-4864 and the existence of a micromolar benzodiazepine binding site in rat brain.” This research, published in Psychopharmacology (Volume 82, Issue 3, Pages 199–202), highlighted the complexity of benzodiazepine binding sites. The study’s DOI was 10.1007/BF00427773, and it can be found under PMID 6326177, with S2CID 28892522.
- In July 1984, Pellow S and File SE explored the “Behavioural actions of Ro 5-4864,” pondering whether it could be considered a peripheral-type benzodiazepine. Their insights, shared in Life Sciences (Volume 35, Issue 3, Pages 229–240), added a layer of depth to the understanding of this compound’s effects. The research can be revisited with a DOI of 10.1016/0024-3205(84)90106-1, and PMID 6087055.
- Marangos PJ, Patel J, Boulenger JP, and Clark-Rosenberg R, in their July 1982 publication in Molecular Pharmacology (Volume 22, Issue 1, Pages 26–32), offered a comprehensive “Characterization of peripheral-type benzodiazepine binding sites in the brain using [3H]Ro 5-4864.” This work remains a pivotal reference for benzodiazepine research and bears the PMID 6289073.
- In April 2005, Veiga S, Azcoitia I, and Garcia-Segura LM explored the potential of “Ro5-4864, a peripheral benzodiazepine receptor ligand,” in reducing reactive gliosis and protecting hippocampal hilar neurons from kainic acid excitotoxicity. Their findings were chronicled in the Journal of Neuroscience Research (Volume 80, Issue 1, Pages 129–137), with a DOI of 10.1002/jnr.20430, hdl:10261/72513, and PMID 15696538. S2CID 23955844 also provides additional context.
- In November 2005, Leonelli E, Yague JG, Ballabio M, Azcoitia I, Magnaghi V, Schumacher M, and collaborators made a remarkable revelation with “Ro5-4864, a synthetic ligand of peripheral benzodiazepine receptor,” showcasing its potential in reducing aging-associated myelin degeneration in the sciatic nerve of male rats. This groundbreaking research was enshrined in Mechanisms of Ageing and Development (Volume 126, Issue 11, Pages 1159–1163) and retains a DOI of 10.1016/j.mad.2005.06.001, hdl:10261/72157, and PMID 16045970, along with S2CID 45797879.
- In July 2007, Azarashvili T, Grachev D, Krestinina O, Evtodienko Y, Yurkov I, Papadopoulos V, and Reiser G revealed the involvement of “The peripheral-type benzodiazepine receptor in the control of Ca2+-induced permeability transition pore opening in rat brain mitochondria.” This remarkable study, found in Cell Calcium (Volume 42, Issue 1, Pages 27–39), is still a valuable resource, with a DOI of 10.1016/j.ceca.2006.11.004 and PMID 17174393.
- In February 2008, Mills C, Makwana M, Wallace A, Benn S, Schmidt H, Tegeder I, and others demonstrated how “Ro5-4864 promotes neonatal motor neuron survival and nerve regeneration in adult rats” in The European Journal of Neuroscience (Volume 27, Issue 4, Pages 937–946). Their research, with a DOI of 10.1111/j.1460-9568.2008.06065.x and PMID 18333964, is a significant contribution to the field and is documented under S2CID 40302403.
- Soustiel JF, Zaaroor M, Vlodavsky E, Veenman L, Weizman A, and Gavish M presented the “Neuroprotective effect of Ro5-4864 following brain injury” in the Experimental Neurology (Volume 214, Issue 2, Pages 201–208) in December 2008. This research, known for its potential in enhancing brain injury recovery, was tagged with a DOI of 10.1016/j.expneurol.2008.08.008 and PMID 18789929, as well as S2CID 2252586.
- In December 2009, Giatti S, Pesaresi M, Cavaletti G, Bianchi R, Carozzi V, Lombardi R, and their collaborators delved into the “Neuroprotective effects of a ligand of translocator protein-18 kDa (Ro5-4864) in experimental diabetic neuropathy.” Their findings, shared in Neuroscience (Volume 164, Issue 2, Pages 520–529), highlighted the potential of Ro5-4864 in addressing diabetic neuropathy. The research can be revisited with a DOI of 10.1016/j.neuroscience.2009.08.005, hdl:10261/72515, and PMID 19665520, along with S2CID 11551493.
- In April 2004, DalBó S, Nardi GM, Ferrara P, Ribeiro-do-Valle RM, and Farges RC unveiled the “Antinociceptive effects of peripheral benzodiazepine receptors” in Pharmacology (Volume 70, Issue 4, Pages 188–194). This research, with a DOI of 10.1159/000075547, is an essential reference in the realm of pain management and is associated with PMID 15001819, as well as S2CID 1116731.
- Gavioli EC, Duarte FS, Bressan E, Ferrara P, Farges RC, and De Lima TC, in their June 2003 study, discovered the “Antidepressant-like effect of Ro5-4864, a peripheral-type benzodiazepine receptor ligand, in the forced swimming test.” Their findings, published in the European Journal of Pharmacology (Volume 471, Issue 1, Pages 21–26), shed light on the potential of Ro5-4864 in the context of depression. The research is accompanied by a DOI of 10.1016/S0014-2999(03)01789-8 and PMID 12809948.
- In January 2015, Solhjoo S and O’Rourke B presented insights into “Mitochondrial instability during regional ischemia-reperfusion,” which underlies arrhythmias in monolayers of cardiomyocytes. This valuable research, documented in the Journal of Molecular and Cellular Cardiology (Volume 78, Pages 90–99), with a DOI of 10.1016/j.yjmcc.2014.09.024 and PMC 4268014, is a pivotal resource for understanding the role of mitochondria in cardiac health, and it can be revisited under PMID 25268650.
- Obame FN, Zini R, Souktani R, Berdeaux A, and Morin D, in October 2007, unveiled how “Peripheral benzodiazepine receptor-induced myocardial protection is mediated by inhibition of mitochondrial membrane permeabilization.” This research, housed in The Journal of Pharmacology and Experimental Therapeutics (Volume 323, Issue 1, Pages 336–345), explored the cardioprotective potential of peripheral benzodiazepine receptors. The study’s DOI was 10.1124/jpet.107.124255, and PMID 17640950 was attached, along with S2CID 12215031.
- Veenman L, Papadopoulos V, and Gavish M, in their comprehensive review, shed light on the “Channel-like functions of the 18-kDa translocator protein (TSPO),” which includes the regulation of apoptosis and steroidogenesis as part of the host-defense response. This invaluable review, found in Current Pharmaceutical Design (Volume 13, Issue 23, Pages 2385–2405), outlines the multifaceted roles of the 18-kDa translocator protein (TSPO). The research is housed with a DOI of 10.2174/138161207781368710 and is discussed under PMID 17692008.
- Papadopoulos V and Lecanu L, in their September 2009 review, recognized the “Translocator protein (18 kDa) TSPO” as an emerging therapeutic target in neurotrauma. Their insights, featured in Experimental Neurology (Volume 219, Issue 1, Pages 53–57), highlight the potential of TSPO as a target for neurotrauma treatments. The review can be revisited with a DOI and PMID 19409385, with PMC 2728790 providing further information.
- In April 2010, Xiao J, Liang D, Zhang H, Liu Y, Li F, and Chen YH explored the potential of “4′-Chlorodiazepam,” a translocator protein (18 kDa) antagonist, in improving cardiac functional recovery during postischemia reperfusion in rats. This research, published in the Experimental Biology and Medicine (Volume 235, Issue 4, Pages 478–486), showcases the potential of 4′-Chlorodiazepam in the context of cardiac health. The study bears a DOI of 10.1258/ebm.2009.009291 and is documented under PMID 20407080, along with S2CID 6403616.