- 1 Tips for Purchasing 4-MPD Online Safely and Effectively
- 2 Why Buy 4-MPD Online?
- 3 Key Tips for Selecting Reliable 4-MPD Vendors
- 4 Advantages of Purchasing 4-MPD Online
- 5 Final Thoughts
- 6 Summary
- 7 FAQ
- 7.1 1. What is 4-Methylpentedrone?
- 7.2 2. What are its other names?
- 7.3 3. How does 4-Methylpentedrone work?
- 7.4 4. Is it legal?
- 7.5 5. What are the effects of 4-methylpentedrone?
- 7.6 6. Is 4-Methylpentedrone safe?
- 7.7 7. Can it be addictive?
- 7.8 8. How is it typically used?
- 7.9 9. Are there any legal alternatives to 4-Methylpentedrone?
- 7.10 10. What precautions should I take if I choose to use it?
- 8 References
Tips for Purchasing 4-MPD Online Safely and Effectively
When exploring options to buy 4-MPD, it’s essential to stay informed and mindful of safe purchasing practices. Whether you’re searching for “buy 4-MPD USA” or “4-MPD for sale,” focusing on trusted vendors and products can make a significant difference in your experience. Here are some actionable tips to help you make the most informed choices when purchasing 4-MPD online.
Why Buy 4-MPD Online?
Online platforms simplify the process of purchasing 4-MPD, offering access to a wide range of products and vendors. With terms like “4-MPD buy” and “purchase 4-MPD” gaining popularity, buyers are increasingly drawn to the convenience, variety, and competitive pricing available online.
Benefits of Purchasing 4-MPD Online
- Global Accessibility
From “4-MPD for sale USA” to “4-MPD Canada,” online vendors ensure availability across regions, catering to a broader audience.
- Ease of Comparison
Platforms advertising “buy 4-MPD online” allow you to compare multiple products and vendors, helping you find the best fit for your needs.
- Discreet Shopping
Reliable platforms prioritize secure packaging and confidential transactions, making the process seamless and private.
Key Tips for Selecting Reliable 4-MPD Vendors
Choosing the right vendor is crucial to ensure product quality and safety. Follow these guidelines to identify trusted sellers in the competitive 4-MPD online market:
- Prioritize Verified Vendors
Look for “4-MPD shop” options with verified user reviews and a strong reputation. Trustworthy vendors often highlight transparency regarding product quality.
- Evaluate Product Descriptions
Check product listings labeled as “4-MPD research chemicals” or “4-MPD buy online” for clear details on purity levels and specifications.
- Confirm Payment and Shipping Security
Reputable sellers provide secure payment options and discreet shipment solutions, ensuring a hassle-free process from checkout to delivery. Sellers focused on “4-MPD USA” or “4-MPD Canada” often offer regional logistics insights.
- Compare Pricing Across Vendors
Terms like “4-MPD sale” or “4-MPD shop” often lead to competitive offers. Compare prices carefully to avoid overpaying while ensuring quality.
Advantages of Purchasing 4-MPD Online
Buying 4-MPD online comes with several benefits beyond convenience. Consider these key advantages as you finalize your choice:
- Diverse Product Range
Vendors offer a wide variety of forms and quantities, appealing to both first-time buyers and regular users.
- Time-Saving Options
Online shopping eliminates the need for in-person visits, making it easier to secure “4-MPD for sale” from the comfort of your home.
Final Thoughts
When purchasing 4-MPD, your best strategy is to focus on informed decision-making. By selecting trusted vendors, verifying product specifications, and comparing prices effectively, you can enjoy the benefits of a seamless online buying experience. Explore “4-MPD research chemicals,” “buy 4-MPD USA,” or “4-MPD for sale” options today to find the best products suited to your requirements.
Summary
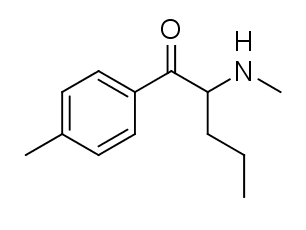
4-Methylpentedrone, also recognized as 4-MPD and 4-Methyl-α-methylamino-valerophenone, is a stimulant substance classified within the cathinone group. This compound has been made available for purchase online as a designer drug. It is structurally similar to 4-methylmethcathinone (mephedrone) and 4-methylbuphedrone (4-MeMABP), with an additional methyl group compared to pentedrone. Alternatively, it can be regarded as the methylamino counterpart of pyrovalerone.
It is important to note that a related compound called 4-methyl-α-ethylaminopentiophenone (4-MEAP) has been mistakenly sold under the name 4-methylpentedrone.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1373918-61-6 |
|---|---|
| ChemSpider | 58951581 |
| UNII | G83HJ935NV |
| Chemical and physical data | |
| Formula | C13H19NO |
| Molar mass | 205.301 g·mol−1 |
FAQ
1. What is 4-Methylpentedrone?
- 4-Methylpentedrone, or 4-MPD, is a stimulant drug in the cathinone class. It is often sold as a designer drug.
2. What are its other names?
- Besides 4-MPD, it is also known as 4-Methyl-α-methylamino-valerophenone.
3. How does 4-Methylpentedrone work?
- 4-Methylpentedrone is believed to work by affecting the release, reuptake, and inhibition of certain neurotransmitters, such as dopamine, norepinephrine, and serotonin, in the brain.
4. Is it legal?
- The legal status of 4-Methylpentedrone varies from country to country and can change over time. It may be considered illegal in some regions due to its classification as a designer drug.
5. What are the effects of 4-methylpentedrone?
- The effects of 4-methylpentedrone can include increased energy, alertness, and euphoria. It may also increase heart rate and blood pressure, and potential side effects like anxiety and insomnia.
6. Is 4-Methylpentedrone safe?
- The safety of 4-methylpentedrone has not been extensively studied, and its use carries risks. It may lead to health problems, addiction, or adverse reactions. It is crucial to exercise caution and avoid use without proper medical guidance.
7. Can it be addictive?
- Like many stimulant drugs, 4-methylpentedrone has the potential to be addictive. Users may develop tolerance and dependence with regular or excessive use.
8. How is it typically used?
- 4-Methylpentedrone is often consumed orally, nasally (snorted), or occasionally by other routes. The method of use can affect the onset and duration of its effects.
9. Are there any legal alternatives to 4-Methylpentedrone?
- It is essential to emphasize that designer drugs or their alternatives can be unsafe and legally questionable. Seeking legal and safer alternatives is recommended.
10. What precautions should I take if I choose to use it?
– If you decide to use 4-methylpentedrone, it’s vital to exercise extreme caution and be aware of potential risks. Start with a low dose, avoid mixing with other substances, and monitor your health closely.
References
- “4-Methylpentedrone”. Cayman Chemical. Retrieved 28 June 2015.
- Hamby D, Burnett A, Jablonsky M, Twamley B, Kavanagh PV, Gardner EA (May 2015). “Identification of 2-(ethylamino)-1-(4-methylphenyl)-1-pentanone (4-MEAP), a New “Legal High” Sold by an Internet Vendor as 4-Methyl Pentedrone”. Journal of Forensic Sciences. 60 (3): 721–6. doi:10.1111/1556-4029.12712. PMID 25923458. S2CID 19336157.
- Uchiyama N, Matsuda S, Kawamura M, Shimokawa Y, Kikura-Hanajiri R, Aritake K, et al. (October 2014). “Characterization of four new designer drugs, 5-chloro-NNEI, NNEI indazole analog, α-PHPP and α-POP, with 11 newly distributed designer drugs in illegal products”. Forensic Science International. 243: 1–13. doi:10.1016/j.forsciint.2014.03.013. PMID 24769262.
