Explore Reliable Options to Order Butylone Online
Finding high-quality Butylone doesn’t have to be a challenge. This sought-after research chemical is widely available through reputable vendors online, making it easier than ever to access what you need. Buyers can confidently browse Butylone for sale, secure in the knowledge that trusted sources prioritize quality and safety.
Why Choose to Buy Butylone Online?
When you opt to buy Butylone online, you’re opening the door to a world of convenience and accessibility. Online platforms allow you to explore options based on your location, whether you need Butylone in the USA, Butylone in Canada, or even globally.
Trusted Butylone vendors often provide detailed product descriptions and transparency about quality. Whether you’re ordering for research purposes or the chemical’s known applications, these sellers prioritize delivering a reliable experience every time.
Benefits of Buying Butylone Online:
- Wider accessibility: Reputable vendors cater to various locations, with specialized services for Butylone USA and Butylone Canada buyers.
- Easy comparison: With multiple Butylone shops online, it’s easier to compare product quality, prices, and customer reviews to make informed decisions.
- Discreet shipping: Most vendors offer secure and discreet packaging, so your purchase is handled with care.
Finding the Right Butylone Vendors
When you’re ready to purchase Butylone, choosing the right vendor is crucial. Look for platforms that emphasize integrity and customer support, whether you’re searching for Butylone for sale or looking to purchase Butylone in bulk.
Key factors to consider include clear pricing details, secure payment methods, and verified customer reviews. The best Butylone vendors also prioritize customer health by offering products labeled with accurate chemical information, ensuring safe experiences.
Order Butylone with Confidence
If you’re ready to make your Butylone buy, consider allocating time to research various online options. Look for vendors showcasing Butylone sale offers or specialized Butylone shop experiences that fit your needs. The right platform will streamline your ordering process, ensuring you get a high-quality product delivered to your doorstep.
Reliable services allow you to order Butylone for personal or professional use without unnecessary hassle. Prioritize clear communication with your chosen vendor to align your expectations and ensure a smooth purchasing experience.
By selecting a trusted source, you can count on a dependable transaction, helping you focus more on your endeavors and less on the uncertainties of online purchase.
Summary
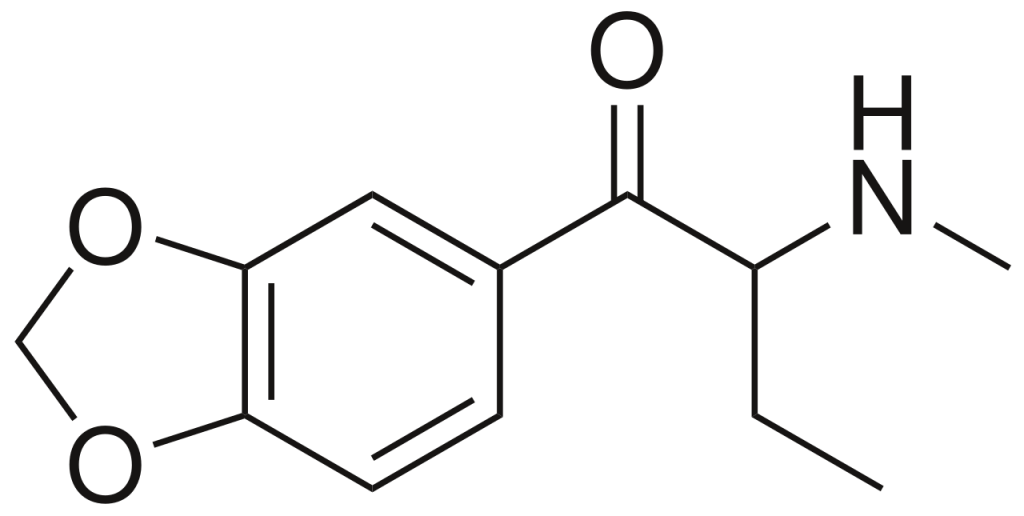
Butylone, alternatively referred to as β-keto-N-methylbenzodioxolylbutanamine (βk-MBDB), is a psychoactive substance belonging to the phenethylamine chemical class. It possesses entactogenic, psychedelic, and stimulant properties. Butylone is recognized as the β-keto derivative, akin to substituted cathinone, of MBDB, and it serves as the substituted methylenedioxyphenethylamine analog of buphedrone.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 802575-11-7 |
|---|---|
| PubChem CID | 56843046 |
| ChemSpider | 21073070 |
| UNII | X72T4EQ4FQ |
| Chemical and physical data | |
| Formula | C12H15NO3 |
| Molar mass | 221.256 g·mol−1 |
History
The initial synthesis of Butylone is credited to Koeppe, Ludwig, and Zeile, as documented in their 1967 research paper. Despite its early origins in academia, it remained relatively unknown until 2005, when it emerged as a designer drug. Notably, Butylone is structurally analogous to MBDB, similar to how methylone relates to MDMA, commonly known as “Ecstasy.” Formal research on Butylone began in 2009, revealing that it undergoes metabolic processes akin to other related drugs such as methylone.
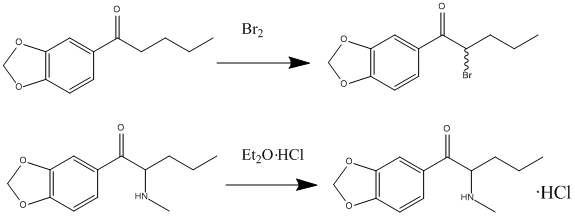
Synthesis
Butylone can be synthesized in a laboratory using the following procedure:
- Begin with 3,4-methylenedioxybutyrophenone, which is dissolved in dichloromethane, and then introduce bromine to obtain 3′,4′-methylenedioxy-2-bromobutyrophenone.
- Dissolve this product in dichloromethane and add it to an aqueous methylamine solution (40%). Subsequently, introduce hydrochloric acid (HCl).
- Remove the aqueous layer and adjust its pH to alkaline by adding sodium bicarbonate.
- To extract the amine, employ ether as a solvent.
- Finally, to obtain butylone, combine a drop of ether with an HCl solution.
Metabolism
Three major metabolic pathways for bk-MBDB are illustrated in the diagram. As a result of demethylation followed by O-methylation, bk-MBDB undergoes metabolism in human urine, leading to the formation of 4-OH-3-MeO and 3-OH-4-MeO metabolites.
The second pathway involves the reduction of the β-ketone, resulting in metabolites with reduced β-ketone functionality.
The third pathway entails N-dealkylation, yielding N-dealkyl metabolites.
It’s important to note that the first two pathways are more prevalent than the third pathway. The most frequently occurring metabolite is the 4-OH-3-MeO metabolite. Metabolites containing a hydroxyl group are typically excreted in urine in their conjugated form.
Mechanism of action
| NET IC50 (μM) | 2.02 (1,5-2,7) |
|---|---|
| DAT IC50 (μM) | 2,90 (2,5-3,4) |
| SERT IC50 (μM) | 6,22 (4,3-9,0) |
| DAT EC50 (μM) | >100 |
|---|---|
| SERT EC50 (μM) | 5,5 (1,8-17) |
Drug prohibition laws
China: As of October 2015, Butylone became a controlled substance in China.
Sweden: In Sweden, Sveriges riksdag classified butylone as a Schedule I narcotic under the category of substances, plant materials, and fungi with no recognized medical use, effective February 1, 2010. This classification was published in the Medical Products Agency’s regulation LVFS 2022:48 and is listed as Butylon, 1-(1,3-bensodioxol-5-yl)-2-(metylamino)butan-1-on.
United States: In the United States, Butylone is classified as a Schedule I controlled substance under the Controlled Substances Act.
FAQ
1. What is Butylone?
Butylone is a synthetic drug belonging to the substituted cathinone class. It is often considered a designer drug known for its stimulant and entactogenic properties.
2. What are the effects of Butylone?
The effects of Butylone can include increased energy, heightened alertness, and a sense of euphoria. It may also enhance sociability and sensory perception. However, it’s important to note that individual reactions may vary.
3. Is Butylone legal?
The legal status of Butylone varies by country and jurisdiction. In many places, it is classified as a controlled substance, making its possession, sale, or use illegal without proper authorization.
4. Are there health risks associated with Butylone use?
The long-term health risks of Butylone are poorly understood due to limited research. However, like other stimulant drugs, it can potentially lead to increased heart rate, blood pressure, and the risk of addiction or psychological dependence.
5. Is Butylone addictive?
Butylone, like many stimulant substances, has the potential for psychological dependence or addiction. Regular and excessive use can lead to cravings and withdrawal symptoms.
6. How is Butylone typically consumed?
Butylone is often ingested orally, commonly as a pill or capsule. Some individuals may also insufflate (snort) it, although this method can be harsh on the nasal passages.
7. Can Butylone be used safely?
Due to its limited research and potential health risks, using Butylone is not recommended. The safest choice is to avoid its use altogether.
References
- Uchiyama N, Kikura-Hanajiri R, Kawahara N, Goda Y (October 2008): This study, published in Japanese, focuses on the analysis of designer drug components detected in products purchased during the fiscal year 2006. It employs NMR as a central analytical technique.
- Zaitsu K, Katagi M, Kamata HT, Kamata T, Shima N, Miki A, et al. (July 2009): In this research, the metabolites of the new designer drugs bk-MBDB and bk-MDEA are determined in human urine. The study provides insights into the metabolism of these substances.
- López-Arnau R, Martínez-Clemente J, Pubill D, Escubedo E, Camarasa J (September 2012): This study explores the comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone, and methylone, shedding light on their effects on the nervous system.
- Prosser JM, Nelson LS (March 2012): A comprehensive review of the toxicology of bath salts, including synthetic cathinones. The paper discusses the health risks associated with these substances.
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A (June 2013): This research investigates the interactions of substituted methcathinones with transporters and receptors, providing insights into their pharmacological properties.
- Saha K, Li Y, Holy M, Lehner KR, Bukhari MO, Partilla JS, et al. (March 2019): The study examines the synthetic cathinones butylone and pentylone, highlighting their actions as dopamine transporter blockers and serotonin transporter substrates.
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, et al. (January 2013): This research involves the pharmacological characterization of designer cathinones in vitro, offering valuable data on their properties.
- “关于印发《非药用类麻醉药品和精神药品列管办法》的通知” (in Chinese, September 2015): This is an official notification from the China Food and Drug Administration regarding the regulation of non-medicinal narcotics and psychotropic substances, including butylone.
- Christina Rångemark Åkerman (29 July 2022, in Swedish): This document discusses amendments to regulations related to controlled substances in Sweden, including butylone, as published by LVFS (Läkemedelsverkets föreskrifter).
