- 1 Summary
- 2 Trans -CO2Me group
- 3 FAQ
- 3.1 1. What is Dichloropane?
- 3.2 2. What are the other names for Dichloropane?
- 3.3 3. How does Dichloropane work?
- 3.4 4. What are the IC50 values for Dichloropane’s reuptake inhibition?
- 3.5 5. How does Dichloropane compare to cocaine?
- 3.6 6. What is the precursor for the synthesis of Dichloropane?
- 3.7 7. Has Dichloropane been used in clinical trials or approved for medical use?
- 3.8 8. Is Dichloropane legal?
- 3.9 9. Are there any known side effects or health risks associated with Dichloropane?
- 4 References
Summary
Dichloropane, also known as (-)-2β-Carbomethoxy-3β-(3,4-dichlorophenyl)tropane or RTI-111, belongs to the phenyltropane class of stimulants. It functions as a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI), exhibiting inhibitory activity with IC50 values of 3.13, 0.79, and 18 nM for serotonin, norepinephrine, and dopamine reuptake, respectively. In animal research, dichloropane demonstrates a slower onset and a prolonged duration of action when compared to cocaine.
It’s worth noting that Methylecgonidine serves as the immediate precursor for synthesising this compound.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 146725-34-0 |
|---|---|
| PubChem CID | 127024 |
| ChemSpider | 112783 |
| UNII | V7SQE82R87 |
| CompTox Dashboard (EPA) | DTXSID20932894 |
| Chemical and physical data | |
| Formula | C15H17Cl2NO2 |
| Molar mass | 314.21 g·mol−1 |
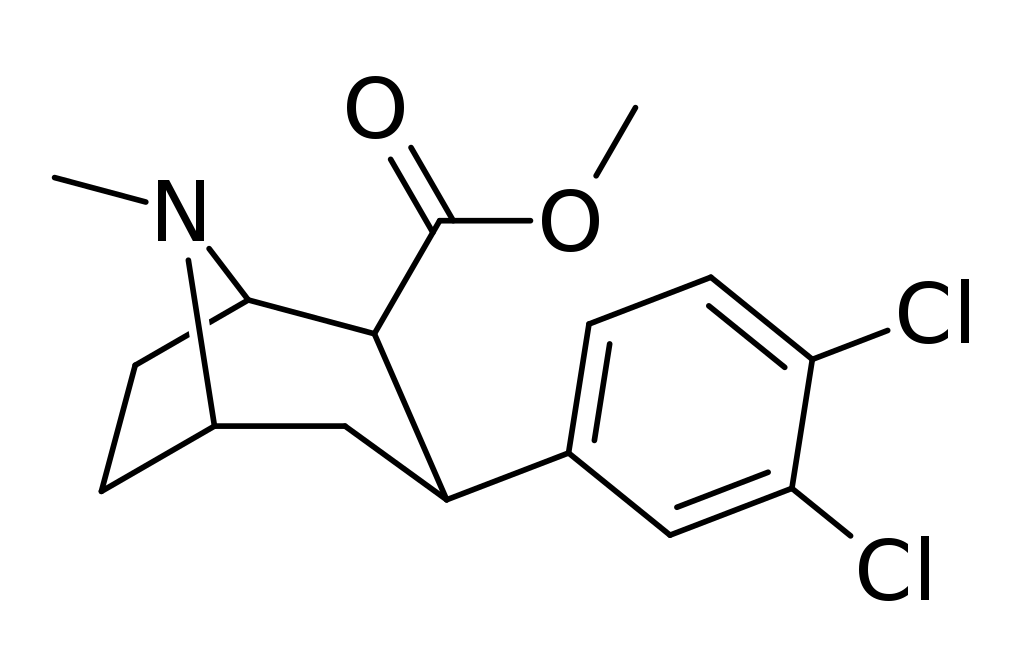
Trans -CO2Me group
The thermodynamic isomer featuring a trans-CO2Me group retains its activity. Neurosearch employed this isomer to synthesize three distinct phenyltropanes that underwent clinical trials:
- Tesofensine
- Brasofensine
- NS-2359 (also known as GSK-372,475)
FAQ
1. What is Dichloropane?
- Dichloropane is a chemical compound belonging to the phenyltropane class. It is known for its stimulant properties and ability to inhibit serotonin, norepinephrine, and dopamine reuptake.
2. What are the other names for Dichloropane?
- Dichloropane is also called (-)-2β-Carbomethoxy-3β-(3,4-dichlorophenyl)tropane, RTI-111, and O-401.
3. How does Dichloropane work?
- Dichloropane acts as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), inhibiting the reuptake of these neurotransmitters in the brain. This action increases these neurotransmitters’ levels in the synapses, resulting in stimulant effects.
4. What are the IC50 values for Dichloropane’s reuptake inhibition?
- Dichloropane has IC50 values of 3.13 nM for serotonin reuptake inhibition, 0.79 nM for norepinephrine reuptake inhibition, and 18 nM for dopamine reuptake inhibition.
5. How does Dichloropane compare to cocaine?
- In animal studies, Dichloropane is reported to have a slower onset and a longer duration of action when compared to cocaine. This characteristic may have implications for its potential use and effects.
6. What is the precursor for the synthesis of Dichloropane?
- Methylecgonidine serves as the direct precursor for the synthesis of Dichloropane.
7. Has Dichloropane been used in clinical trials or approved for medical use?
- As of my last knowledge update in September 2021, Dichloropane had not been approved for medical use, and information about its clinical trials was limited. Please consult the latest sources for any updates on its status.
8. Is Dichloropane legal?
- The legal status of Dichloropane may vary by country and region. It is essential to check your area’s specific laws and regulations regarding the possession, sale, or use of this substance.
9. Are there any known side effects or health risks associated with Dichloropane?
- Information on the safety profile and potential health risks of Dichloropane may be limited. It is crucial to exercise caution and consult with healthcare professionals if you have concerns about its use.
References
- Carroll FI, Blough BE, Nie Z, Kuhar MJ, Howell LL, Navarro HA (April 2005). “Synthesis and monoamine transporter binding properties of 3beta-(3′,4′-disubstituted phenyl)tropane-2beta-carboxylic acid methyl esters”. This article can be found in the Journal of Medicinal Chemistry, Volume 48, Issue 8, pages 2767–71. The DOI is 10.1021/jm040185a, and the PMID is 15828814.
- Ranaldi R, Anderson KG, Carroll FI, Woolverton WL (December 2000). “Reinforcing and discriminative stimulus effects of RTI 111, a 3-phenyltropane analog, in rhesus monkeys: interaction with methamphetamine”. This research is published in Psychopharmacology, Volume 153, Issue 1, pages 103–10, with a DOI of 10.1007/s002130000602 and a PMID of 11255920. S2CID 29716872.
- Cook CD, Carroll IF, Beardsley PM (December 2001). “Cocaine-like discriminative stimulus effects of novel cocaine and 3-phenyltropane analogs in the rat”. This study can be found in Psychopharmacology, Volume 159, Issue 1, pages 58–63, with a DOI of 10.1007/s002130100891 and a PMID of 11797070. S2CID 25696981.
- Carroll FI, Mascarella SW, Kuzemko MA, Gao Y, Abraham P, Lewin AH, et al. (September 1994). “Synthesis, ligand binding, and QSAR (CoMFA and classical) study of 3 beta-(3′-substituted phenyl)-, 3 beta-(4′-substituted phenyl)-, and 3 beta-(3′,4′-disubstituted phenyl)tropane-2 beta-carboxylic acid methyl esters”. This research is featured in the Journal of Medicinal Chemistry, Volume 37, Issue 18, pages 2865–73, with a DOI of 10.1021/jm00044a007 and a PMID of 8071935.
