Beautiful Plants For Your Interior
Summary
βk-2C-B, also known as Bk-2C-B, is a recently developed psychedelic compound. This novel substance is structurally related to 2C-B, belonging to the 2C family of psychedelics. Typically consumed orally, βk-2C-B is used for recreational purposes. Notably, it is classified as a controlled substance in several countries, including Canada, Germany, Switzerland, and the United Kingdom.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 807631-09-0 |
|---|---|
| PubChem CID | 11288812 |
| ChemSpider | 9463799 |
| UNII | W6KJ195I24 |
| Chemical and physical data | |
| Formula | C10H12BrNO3 |
| Molar mass | 274.111 g/mol (freebase) 310.572 g/mol (HCl salt) g·mol−1 |
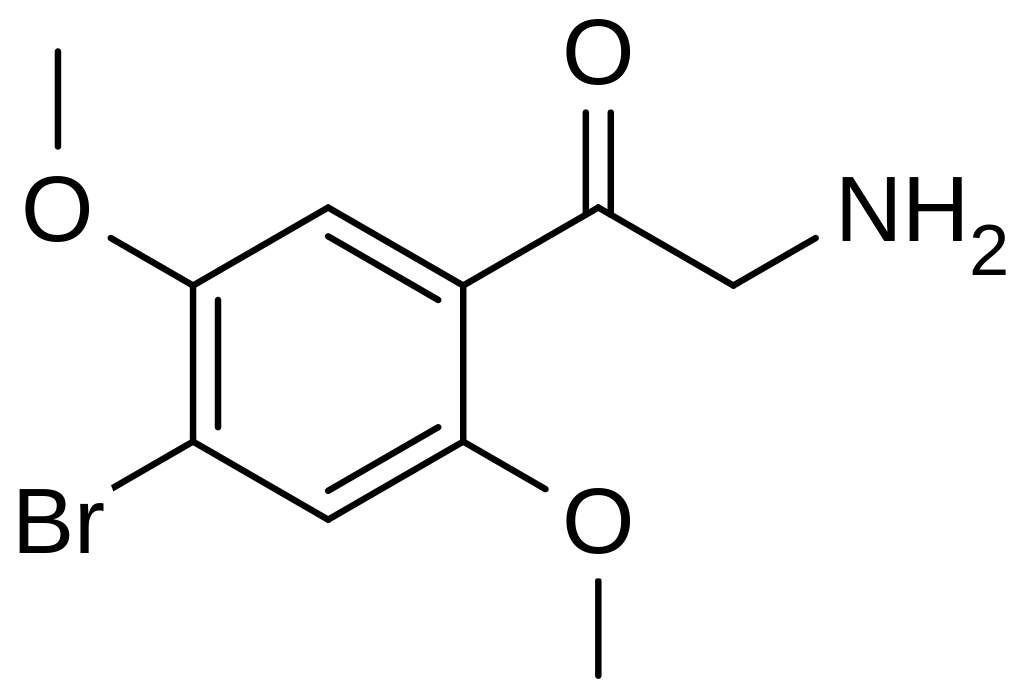
History
βk-2C-B, categorized as a designer drug, is specifically identified as the beta-keto analog of the controlled substance 2C-B, which Alexander Shulgin originally synthesized. The origin of βk-2C-B remains unknown, but it entered the market as a recreational drug around mid-2013. While an earlier synthesis of this compound had been documented, scientific papers providing analytical characterizations of βk-2C-B began to emerge in the years following its introduction. This substance is accessible online and is commonly referred to as a psychedelic drug. Notably, as of October 12, 2016, βk-2C-B has been classified as a controlled substance (Schedule III) in Canada. It is also illegal in Germany, Switzerland, and the United Kingdom.
Chemistry
βk-2C-B belongs to the substituted phenethylamine class of compounds. This substance is characterized by methoxy substitutions at the 2- and 5-positions on the ring, along with a bromine atom at the 4-position. Notably, it features a ketone group at the beta (β) position, adjacent to the functional amine group linked to the alpha (α) carbon. This structural arrangement gives it the name that highlights its resemblance to 2C-B. Due to the presence of the ketone functional group, it is accurately classified as a ketophenethylamine. It is important to clarify that despite certain misconceptions, βk-2C-B is not a substituted cathinone; this is because cathinone are ketoamphetamines, whereas βk-2C-B does not belong to the amphetamine group.
Synthesis
The synthetic procedure for βk-2C-B, as reproduced by Power et al., follows a series of steps originating from 4-bromo-2,5-dimethoxybenzaldehyde:
- Initiation with a Grignard reaction using methylmagnesium bromide.
- Oxidation typically employs reagents like pyridinium chlorochromate (PCC).
- Introduction of an α-bromine atom.
- Reaction with hexamethylenetetramine.
- Finalization with acid hydrolysis.
The mechanism of this synthetic route commences with a nucleophilic attack on the ketone carbon of the initial compound, yielding a secondary alcohol. The use of a potent oxidizing agent like PCC then converts this secondary alcohol back into a ketone. The primary carbon in close proximity is easily brominated via ketone halogenation. Subsequently, a Delépine reaction is utilized to transform the alkyl halide into a primary amine, ultimately yielding βk-2C-B in the form of a mixed hydrochloride/hydrobromide salt.
The Delépine reaction stands out due to its simplicity, brief reaction times, and reliance on readily available and cost-effective reagents and equipment.
Reactions
Dimerization
The primary amine present in βk-2C-B can undergo a reversible reaction with the beta ketone of another βk-2C-B molecule and vice versa, leading to the formation of two imine bonds between the molecules. In this process, water is eliminated. The extent of imine formation largely depends on the pH of the environment, with optimal results observed at around pH 5. At higher pH levels, there may not be sufficient acid to protonate the hydroxyl group. In comparison, at lower pH levels, most of the amine reactant becomes protonated, consequently losing its nucleophilic properties necessary for the reaction.
Nevertheless, experimental evidence has shown that intramolecular reaction readily proceeds for βk-2C-B under neutral pH conditions. When imine formation reaches its full extent between two βk-2C-B molecules, it results in a purple pyrazine dimer. This dimer can be reversed to βk-2C-B through imine hydrolysis, which can be catalyzed by acid, although it’s not an absolute requirement.
Pyrolysis
The thermal decomposition of βk-2C-B has been studied using a simulated ‘meth pipe’ scenario. During this process, twelve major pyrolysis products were identified. Some of these compounds are known to have toxic properties, particularly the α-chloro ketones, while others are considered safe. Interestingly, certain analogues of these compounds have even found applications in medicine. For instance, 1-bromo-4-(2-bromoethyl)-2,5-dimethoxybenzene, one of the pyrolysis products, has been patented for its suitability in treating glaucoma.
Pharmacology and toxicology
- Structure-activity relationships (SAR): The chemical structure of βk-2C-B facilitates various interactions between the compound and target receptors within the body. The functional groups present in the structure have distinct pharmacological activities at receptor sites. Key SAR aspects for βk-2C-B include:
- a. Hydrogen bonding of the primary amine: The primary amine exhibits both hydrogen bond donor and acceptor properties. However, protonation can occur upon interaction with target binding sites, leading to ionization and a loss of hydrogen bond acceptor capacity.
- b. Dipole-dipole interaction of the β-ketone: The permanent dipole moment originating from the ketone group allows for van der Waals dipole-dipole attractions between the compound and target binding sites. The carbonyl oxygen’s lone pairs also enable hydrogen bonding.
- c. Hydrophobic interactions of the aromatic ring: The planar aromatic ring structure in βk-2C-B’s phenethylamine backbone can engage in hydrophobic interactions with flat hydrophobic regions of target binding sites. The ring’s substituted methoxy groups contribute to van der Waals interactions and possess hydrogen bond acceptor properties.
- d. Interactions of the bromine: The polar hydrophobic nature of the bromine atom enhances its affinity for receptors. Additionally, the electronegativity of bromine extends the electron cloud of nucleophilic binding sites.
- Binding affinities: βk-2C-B, like other substituted cathinones of the phenethylamine family, is presumed to exhibit affinity for various serotonin 5-HT2 receptor subtypes. Potential interference with the reuptake of dopamine, serotonin, and noradrenaline is also plausible. Although specific receptor affinities are not well-documented for βk-2C-B, it is known that the introduction of the β-ketone group significantly reduces its binding affinity at the 5-HT2A receptor in comparison to 2C-B. The EC50 and efficacy for βk-2C-B in relation to β-arrestin2 recruitment to the 5-HT2A receptor are 905 nM and 40.8%, respectively, whereas 2C-B demonstrates an EC50 of 9.03 nM and an efficacy of 89.0%. Furthermore, at a concentration of 270 nM, βk-2C-B has been observed to elicit a 0 to 50% agonistic response at the μ-opioid receptor. Lastly, βk-2C-B exhibits weak inhibition of monoamine oxidase (MAO) enzymes, with an IC50 value of 14 μM for MAO-B and an undetermined IC50 value for MAO-A due to even weaker inhibition.
Pharmacokinetics
- General Considerations: Limited knowledge is available regarding the pharmacokinetics of βk-2C-B, primarily due to its status as a novel psychedelic. Consequently, understanding its clearance, biotransformation, and toxicity within the body remains challenging. This absence of information is common for designer drugs, making recreational use inadvisable.
- Possible Biometabolism: Extrapolating from the parent compound, compounds in the 2C family are typically metabolized by liver hepatocytes, involving processes like deamination and demethylation. Oxidative deamination is common, and it can lead to the production of dimethoxybenzoic acid derivatives. However, βk-2C-B’s relatively high dosage, compared to other 2C family members, may imply differing affinities for pharmacologically active sites. This could result in increased toxicity, especially given its extended half-life.
- Interactions and Species Differentiation: The biotransformation enzymes responsible for metabolism can vary significantly between species and sexes, which can lead to species differentiation in metabolizing βk-2C-B. Additionally, βk-2C-B’s inhibition of monoamine oxidase (MAO) may potentiate drugs mimicking amine neurotransmitters, potentially affecting interactions with other substances.
Effects
- Subjective Effects: The lack of substantial research on compounds within the 2C family, including βk-2C-B, means that most information is derived from anecdotal reports by recreational users. Commonly reported effects include open and closed-eye visuals, time distortion, and euphoria, consistent with the experiences associated with other psychedelics in the substituted phenethylamine class.
- Comparison to 2C-B: Users often describe βk-2C-B as more stimulating and less psychedelic when compared to 2C-B. This shift, along with its extended duration, can intensify the perceived effects. Physiologically, increased heart rate and blood pressure are frequently reported. These physiological responses can further enhance the sense of euphoria, perceptual effects (particularly colour intensity), and bodily sensations.
Tolerance
- Non-addictive Nature: βk-2C-B, like other psychedelics, is generally considered non-addictive. With repeated use, individuals will develop tolerance not only to βk-2C-B but also to other psychedelic substances. Cross-tolerance is typical for compounds sharing similar mechanisms of action, such as derivatives of psychedelic phenethylamines.
Legal status
- Canada: As of October 12, 2016, βk-2C-B is classified as a Schedule III controlled substance.
- Germany: In Germany, βk-2C-B is controlled under the New Psychoactive Substances Act (NpSG) as of November 26, 2016. Possession is illegal, although it is not penalized.
- Sweden: βk-2C-B was designated as a narcotic substance on April 5, 2019.
- Switzerland: In Switzerland, βk-2C-B is a controlled substance and is specifically listed under Verzeichnis E.
- United Kingdom: The production, supply, or import of βk-2C-B is illegal in the United Kingdom under the Psychoactive Substance Act, effective as of May 26, 2016.
- United States: In the United States, βk-2C-B is currently unscheduled at the federal level. However, it may be considered an analogue of 2C-B under the Federal Analogue Act, potentially leading to classification as a Schedule I drug. Please note that state laws may also apply, so it is essential to be aware of specific regulations in each state.
Hazards
While there are no specific hazards uniquely associated with βk-2C-B, it’s important to note that certain adverse effects commonly found in 2C-phenethylamines could potentially manifest in βk-2C-B usage. These effects may include chills, nausea, anxiety, confusion, agitation, hypertension, seizures, and, in severe cases, fatalities.
The use of βk-2C-B might induce hallucinations, violent behaviour, and dis-coordination, with potentially severe consequences not only for the individual user but also for the safety of the general public. The severity of these effects is dose-dependent, and the risk tends to escalate with higher doses. Both the United States and the United Kingdom have reported instances of both non-fatal and fatal intoxications associated with 2C-phenethylamines and related substances within this psychoactive family. Therefore, it’s crucial to exercise caution and be well-informed when considering the use of such compounds.
FAQ
1. What is βk-2C-B?
βk-2C-B is a designer drug, specifically the beta-keto analogue of the controlled substance 2C-B. It’s known for its psychedelic properties and is sometimes used recreationally.
2. How does βk-2C-B compare to 2C-B?
βk-2C-B is structurally similar to 2C-B, but it is considered less potent, meaning that higher doses are required to achieve similar effects.
3. How is βk-2C-B used?
It is typically taken orally. Other routes of administration, like insufflation (snorting), are not recommended as they can cause irritation and reduce bioavailability.
4. What is a typical dosage for βk-2C-B?
A threshold dose is reported to be around 50-60 mg, while a common dosage range is 60-150 mg. However, users should be cautious about exceeding 100-120 mg to avoid potential toxic effects.
5. What are the effects of βk-2C-B?
Effects are reported to include open and closed-eye visuals, time distortion, and euphoria. βk-2C-B is often described as more stimulating and less psychedelic compared to 2C-B.
6. Is βk-2C-B addictive?
Like other psychedelics, βk-2C-B is generally considered non-addictive. Frequent use can lead to tolerance.
7. In which countries is βk-2C-B a controlled substance?
βk-2C-B is controlled in several countries, including Canada, Germany, Sweden, Switzerland, and the United Kingdom. In the United States, it is unscheduled but may be considered an analogue of 2C-B under the Federal Analogue Act.
8. Are there safety concerns with βk-2C-B?
Common hazards associated with 2C-phenethylamines, such as chills, nausea, anxiety, confusion, hypertension, seizures, and even death, could potentially be associated with βk-2C-B use. High doses increase the risk of these effects.
9. Is βk-2C-B illegal to purchase online?
The legality of purchasing βk-2C-B online varies by country. Users should be aware of their local laws and regulations regarding this substance.
10. Can βk-2C-B be used for therapeutic purposes?
There is limited research on the therapeutic potential of βk-2C-B. It is primarily used recreationally, and its safety and efficacy for therapeutic applications are not well-established.
References
- Frison G, Odoardi S, Frasson S, Sciarrone R, Ortar G, Romolo FS, Strano Rossi S (July 2015). “Characterization of the designer drug bk-2C-B (2-amino-1-(bromo-dimethoxyphenyl)ethan-1-one) by gas chromatography/mass spectrometry without and with derivatization with 2,2,2-trichloroethyl chloroformate, liquid chromatography/high-resolution mass spectrometry, and nuclear magnetic resonance”. Rapid Communications in Mass Spectrometry. 29 (13): 1196–1204. doi:10.1002/rcm.7211. PMID 26395784.
- Glennon RA, Bondarev ML, Khorana N, Young R, May JA, Hellberg MR, et al. (November 2004). “Beta-oxygenated analogues of the 5-HT2A serotonin receptor agonist 1-(4-bromo-2,5-dimethoxyphenyl)-2-aminopropane”. Journal of Medicinal Chemistry. 47 (24): 6034–6041. doi:10.1021/jm040082s. PMID 15537358.
- Power JD, Kavanagh P, O’Brien J, Barry M, Twamley B, Talbot B, et al. (June 2015). “Test purchase, identification and synthesis of 2-amino-1-(4-bromo-2, 5-dimethoxyphenyl)ethan-1-one (bk-2C-B)”. Drug Testing and Analysis. 7 (6): 512–518. doi:10.1002/dta.1699. PMID 25078895.
- “Canada Gazette – Regulations Amending the Food and Drug Regulations (Part J — 2C-phenethylamines)”. Public Works and Government Services Canada. Government of Canada. 2016-05-04. Retrieved 2021-08-07.
- Kaur N, Kishore D (2013-12-01). “An insight into hexamethylenetetramine: a versatile reagent in organic synthesis”. Journal of the Iranian Chemical Society. 10 (6): 1193–1228. doi:10.1007/s13738-013-0260-2. ISSN 1735-2428. S2CID 93220780.
- Texter KB, Waymach R, Kavanagh PV, O’Brien JE, Talbot B, Brandt SD, Gardner EA (January 2018). “Identification of pyrolysis products of the new psychoactive substance 2-amino-1-(4-bromo-2,5-dimethoxyphenyl)ethanone hydrochloride (bk-2C-B) and its iodo analogue bk-2C-I”. Drug Testing and Analysis. 10 (1): 229–236. doi:10.1002/dta.2200. PMID 28371351.
- Pottie E, Cannaert A, Stove CP (October 2020). “In vitro structure-activity relationship determination of 30 psychedelic new psychoactive substances by means of β-arrestin 2 recruitment to the serotonin 2A receptor”. Archives of Toxicology. 94 (10): 3449–3460. doi:10.1007/s00204-020-02836-w. hdl:1854/LU-8687071. PMID 32627074. S2CID 220337019.
- Åstrand A, Guerrieri D, Vikingsson S, Kronstrand R, Green H (December 2020). “In vitro characterization of new psychoactive substances at the μ-opioid, CB1, 5HT1A, and 5-HT2A receptors-On-target receptor potency and efficacy, and off-target effects”. Forensic Science International. 317: 110553. doi:10.1006/j.forsciint.2020.110553. PMID 33160102.
- Wagmann L, Brandt SD, Stratford A, Maurer HH, Meyer MR (February 2019). “Interactions of phenethylamine-derived psychoactive substances of the 2C-series with human monoamine oxidases”. Drug Testing and Analysis. 11 (2): 318–324. doi:10.1002/dta.2494. PMID 30188017.
- “Åtta nya ämnen klassas som narkotika” [Eight new substances are classified as narcotics]. Folkhälsomyndigheten [The Swedish Public Health Agency] (in Swedish). 5 April 2019. “På förslag av Folkhälsomyndigheten har regeringen beslutat att klassificera ytterligare åtta ämnen som narkotika från och med den 5