Beautiful Plants For Your Interior
Summary
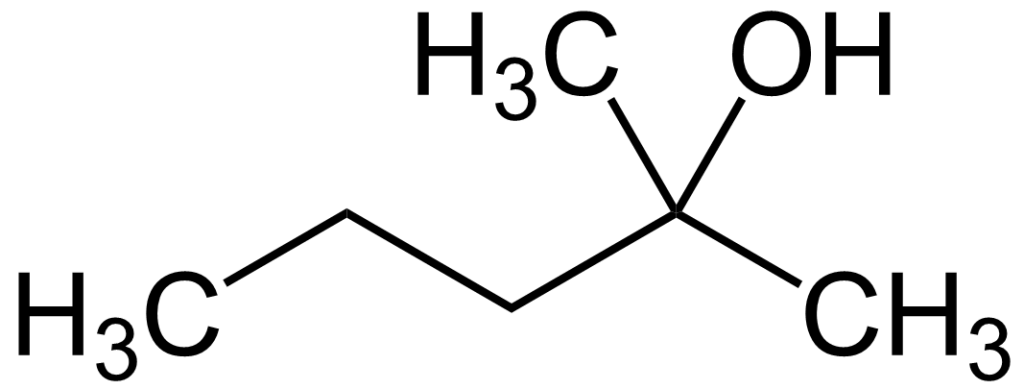
2-Methyl-2-pentanol, also known by its IUPAC name, 2-methylpentan-2-ol, is an organic chemical compound. It finds utility in gas chromatography as an additive for the differentiation of branched compounds, particularly alcohols, due to its unique properties.
Moreover, the detection of 2-Methyl-2-pentanol in urine serves as an indicator for exposure to 2-methylpentane, making it valuable in toxicological assessments.
It is noteworthy that, akin to various other short-chain alcohols, 2-methyl-2-pentanol has the potential to induce intoxication and sedative effects similar to ethanol. However, it should be noted that it can be more irritating to mucous membranes and generally poses a higher level of toxicity to the human body.
| Identifiers | |
|---|---|
| CAS Number | 590-36-3 [PubChem] |
| 3D model (JSmol) | Interactive image |
| ChEMBL | ChEMBL450417 |
| ChemSpider | 11056 |
| ECHA InfoCard | 100.008.802 |
| EC Number | 209-681-0 |
| PubChemCID | 11543 |
| UNII | HU2SO831KP |
| UN number | 2560 |
| CompTox Dashboard(EPA) | DTXSID4060440 |
FAQ
- What is 2-methyl-2-pentanol?
- 2-Methyl-2-Pentanol, also known as 2-methylpentan-2-ol, is an organic chemical compound.
- What is the IUPAC name of 2-methyl-2-pentanol?
- The IUPAC name for 2-methyl-2-pentanol is 2-methylpentan-2-ol.
- What is the primary application of 2-methyl-2-pentanol?
- 2-Methyl-2-Pentanol is commonly used in gas chromatography as an additive to help differentiate between various branched compounds, particularly alcohols, due to its unique chemical properties.
- How can 2-methyl-2-pentanol be utilized in toxicological assessments?
- The presence of 2-methyl-2-pentanol in urine can serve as an indicator of exposure to 2-methylpentane, making it a valuable tool in toxicological assessments.
- What are the potential effects of 2-methyl-2-pentanol on the human body?
- Similar to many other short-chain alcohols, 2-methyl-2-pentanol can induce intoxication and sedative effects resembling ethanol. However, it’s essential to note that it can be more irritating to mucous membranes and generally poses a higher level of toxicity to the human body.
- Is 2-methyl-2-pentanol safe for general use?
- The safety of 2-methyl-2-pentanol depends on its intended use and the precautions taken. Like any chemical compound, it should be handled with care and in accordance with safety guidelines.
- Where can I find more information about 2-methyl-2-pentanol and its applications?
- You can find additional information and detailed research in scientific publications, journals, and academic resources related to organic chemistry and gas chromatography.
References
- In the world of chemistry and physics, Lide DR (1998) delved into the intricacies of the field with the “Handbook of Chemistry and Physics” in its 87th edition. This comprehensive reference, situated in Boca Raton, Florida, offers a wealth of knowledge, spanning from pages 3 to 398 and 8 to 106. The ISBN for this edition is 0-8493-0594-2.
- For those venturing into the realm of quantitative gas chromatography, Guiochon G and Guillemin CL (1988) offer valuable insights in their work, “Quantitative Gas Chromatography: For Laboratory Analyses and On-line Process Control.” This resource, published by Elsevier, encompasses a wealth of information across 518 pages. The ISBN for this publication is 978-0-444-42857-8, and it was retrieved on 2010-01-22.
- Lauwerys RR and Hoet P (2001) provide crucial guidelines for dealing with industrial chemical exposure in their publication, “Industrial Chemical Exposure: Guidelines for Biological Monitoring.” This publication from CRC Press spans 190 pages, providing essential insights. The ISBN for this resource is 978-1-56670-545-5, and it was retrieved on 2010-01-22.
- For a deeper understanding of the interaction between ethanol and the GABA A receptor-gated chloride ion channel, Morrow AL, Montpied P, and Paul SM (1991) explored this topic in “Neuropharmacology of Ethanol.” Situated in Boston, MA., this resource is published by Birkhäuser and spans pages 49 to 76. The DOI for this research is 10.1007/978-1-4757-1305-3_3, and the ISBN is 978-1-4757-1307-7.