Beautiful Plants For Your Interior
Summary
25I-NBMD (NBMD-2C-I, Cimbi-29) was first identified as a derivative of the phenethylamine hallucinogen 2C-I in 2006 by Purdue University researchers under David Nichols’s leadership. Acting as a potent partial agonist for the 5HT2A receptor, it exhibits a Ki of 0.049 nM at the human 5HT2A receptor. Notably, the related 4-bromo analogue, 25B-NBMD, has been instrumental in molecular dynamics studies investigating the structure of the 5-HT2A receptor.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 919797-25-4 |
|---|---|
| PubChem CID | 57507899 |
| ChemSpider | 26234932 |
| UNII | R99110126K |
| CompTox Dashboard (EPA) | DTXSID70726967 |
| Chemical and physical data | |
| Formula | C18H20INO4 |
| Molar mass | 441.265 g·mol−1 |
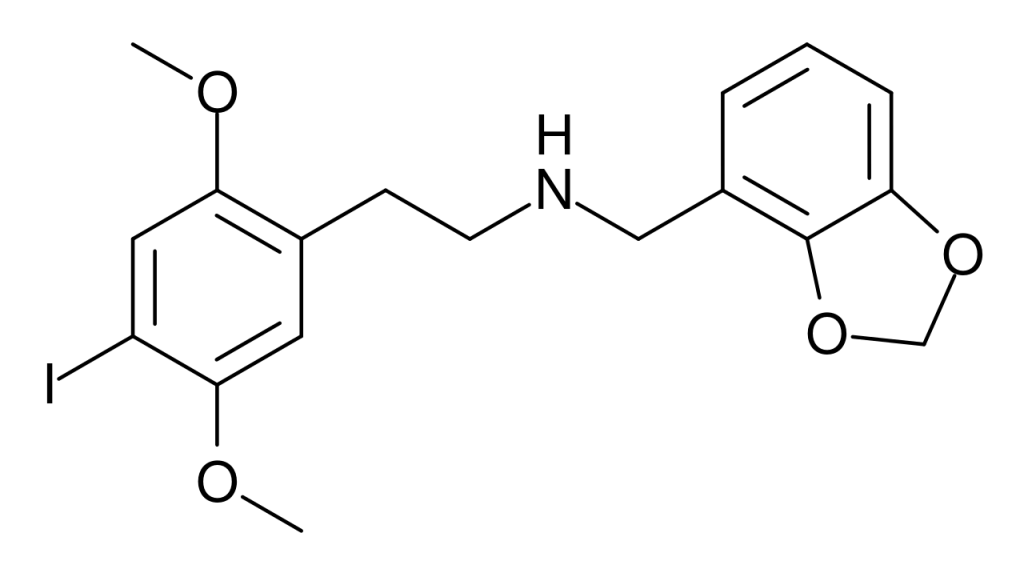
Legality
Sweden
The Swedish Riksdag included 25I-NBMD in the Narcotic Drugs Punishments Act, placing it under Swedish Schedule I, encompassing “substances, plant materials, and fungi which normally do not have medical use.” This addition was made effective as of January 16, 2015, and was published by the Medical Products Agency (MPA) in regulation LVFS 2014:11. The compound is listed as 25I-NBMD, known chemically as 2-(4-jodo-2,5-dimetoxifenyl)-N-[(2,3-metylendioxifenyl)metyl]etanamin.
United Kingdom
In the United Kingdom, 25I-NBMD is classified as a Class A drug per the N-benzyl phenethylamine catch-all clause outlined in the Misuse of Drugs Act 1971.
FAQ
- What is 25I-NBMD?
- 25I-NBMD, also known as NBMD-2C-I or Cimbi-29, is a chemical compound derived from the phenethylamine hallucinogen 2C-I. It was discovered in 2006 by a research team at Purdue University led by David Nichols.
- How does 25I-NBMD affect the body?
- 25I-NBMD acts as a potent partial agonist for the 5HT2A receptor. This receptor is associated with the serotonin system and is crucial in regulating mood, perception, and various cognitive functions.
- What is the pharmacological potency of 25I-NBMD?
- 25I-NBMD has a high pharmacological potency, with a Ki value of 0.049 nM at the human 5HT2A receptor, indicating a strong affinity for this receptor.
- Is 25I-NBMD similar to other compounds like 25B-NBMD?
- Yes, 25I-NBMD is chemically related to 25B-NBMD, its 4-bromo analogue. These compounds are used in molecular dynamics studies to understand the structure and function of the 5-HT2A receptor.
- Is 25I-NBMD legal in Sweden?
- No, 25I-NBMD is not legal in Sweden. It is classified as a narcotic substance under Swedish Schedule I, which includes imports, plant materials, and fungi that do not have medical use. This classification took effect on January 16, 2015.
- Is 25I-NBMD legal in the United Kingdom?
- 25I-NBMD is classified as a Class A drug in the United Kingdom. This classification is due to its inclusion under the N-benzyl phenethylamine catch-all clause in the Misuse of Drugs Act 1971.
References
- Braden MR, Parrish JC, Naylor JC, Nichols DE (December 2006). “Elucidating the molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with highly potent N-benzyl phenethylamine agonists”. Molecular Pharmacology. 70 (6): 1956–64. doi:10.1124/mol.106.028720. PMID 17000863. S2CID 15840304.
- Braden MR (2007). “Advancing towards a comprehensive biophysical understanding of hallucinogen action” (Ph.D. thesis). Purdue University. ProQuest 304838368.
- Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, et al. (April 2011). “Synthesis and in vivo assessment of a range of substituted 11C-phenethylamines as PET tracers for 5-HT (2A) receptor agonists”. European Journal of Nuclear Medicine and Molecular Imaging. 38 (4): 681–93. doi:10.1007/s00259-010-1686-8. PMID 21174090. S2CID 12467684.
- Isberg V, Balle T, Sander T, Jørgensen FS, Gloriam DE (February 2011). “Development of a G protein- and agonist-bound serotonin 5-HT2A receptor model through steered molecular dynamics simulations”. Journal of Chemical Information and Modeling. 51 (2): 315–25. doi:10.1021/ci100402f. PMID 21261291.
- “Regulations amending the Medical Products Agency’s provisions (LVFS 2011:10) on lists of controlled substances” (PDF) (in Swedish). Archived from the original (PDF) on 2015-03-16. Retrieved 2017-04-21.
- “The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014”. www.legislation.gov.uk.
- “Exploring the properties of N-(2C-I)-Fentanyl | PiHKAL · info”. isomerdesign.com.