Beautiful Plants For Your Interior
Summary
2C-E is a member of the 2C family, a group of psychedelic phenethylamines. It was created by Alexander Shulgin and is detailed in his publication “PiHKAL.” Similar to its counterparts within the same chemical family, 2C-E elicits sensory and cognitive effects when interacting with living organisms.
| Identifiers | |
|---|---|
| CAS Number | 71539-34-9 |
| 3D model (JSmol) | Interactive image |
| ChEMBL | ChEMBL124063 |
| ChemSpider | 21106222 |
| ECHA InfoCard | 100.221.016 |
| PubChemCID | 24729233 |
| UNII | I190284UXX |
| CompTox Dashboard(EPA) | DTXSID40221772 |
Properties
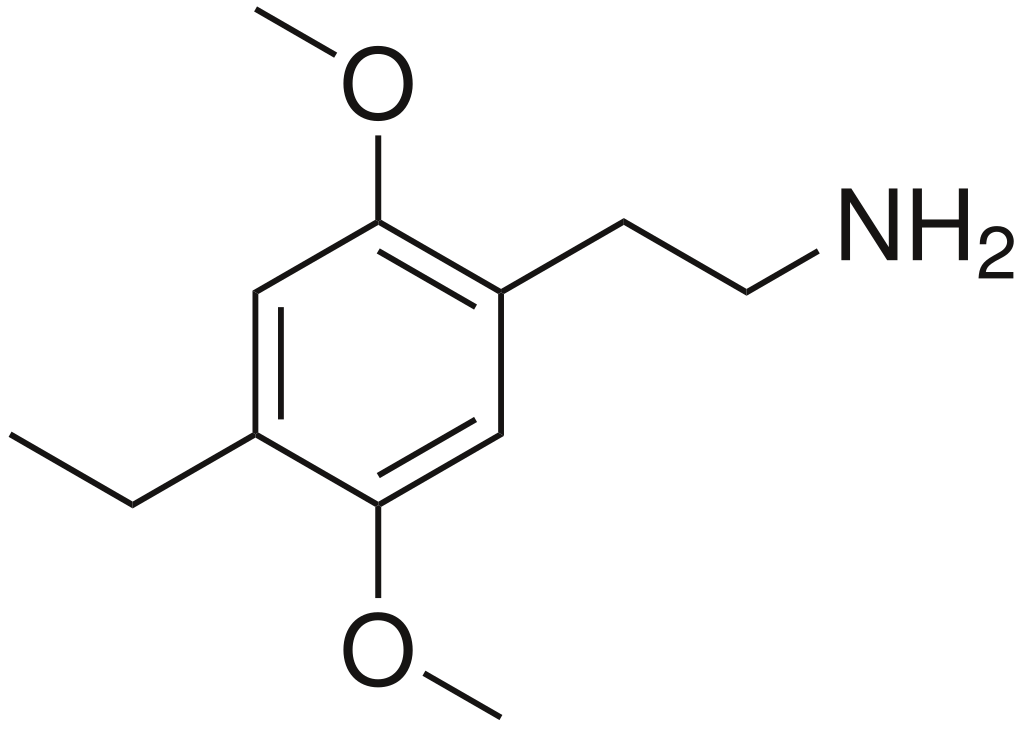
2,5-Dimethoxy-4-ethylphenethylamine exists as a colourless oil. To obtain crystalline forms, the amine salt is produced by reacting the free base with a mineral acid, commonly hydrochloric acid (HCl).
Regarding its properties, Shulgin does not specify an exact boiling point for the free base. He mentions that, during one synthesis, a fraction boiling between 90 and 100 °C at 0.25 mmHg pressure was collected and subsequently transformed into the hydrochloride salt. The melting point of the hydrochloride salt, according to Shulgin, falls within the range of 208.5–210.5 °C.
Effects
As per Shulgin’s observations, the typical duration of 2C-E’s effects spans from six to ten hours when administered at an average dose, with a plateau phase lasting between three to six hours.
2C-E’s effects are often characterized as “neutral” in comparison to other psychedelic compounds, including related 2C-x molecules. In his work “PiHKAL,” Shulgin writes:
“2C-E is another one of the remarkable ‘half-dozen.’ Its range is intentionally broad. At a 10-milligram dosage, users have reported profound experiences, while a young lady’s 30-milligram trial was notably intense and somewhat distressing. My initial encounter with 2C-E was profoundly significant, warranting a dedicated chapter in the story. Several individuals have expressed sentiments such as, ‘I’m not sure if I enjoyed it, as it wasn’t particularly fun, but I plan to explore it again.’ There is an element here that promises rewarding experiences for the experimenter. While we may not fully comprehend the complete nature of 2C-E at the moment, it remains a challenging yet worthwhile substance—a truly valuable material.”
Adverse effects associated with 2C-E encompass tachycardia, hypertension, agitation, delirium, and hallucinations. Notably, there have been at least two reported deaths attributed to 2C-E overdoses.
Drug prohibition laws
Australia
In Queensland, the inclusion of 2C-E in the ‘Dangerous Drugs’ list of the ‘Drugs Misuse Act 1986’ was enacted through the ‘Drugs Misuse Amendment Act 2008,’ rendering it illegal for production, supply, or possession.
Canada
Effective October 31, 2016, Canada classified 2C-E as a controlled substance under Schedule III.
China
As of October 2015, China designated 2C-E as a controlled substance, making its production, supply, and possession illegal.[9]
Denmark
2C-E has been added to the roster of Schedule B controlled substances in Denmark.
Germany
Germany classifies 2C-E as an Anlage I controlled drug.
New Zealand
In New Zealand, a comprehensive Analogues section in Schedule 3 / Class C of their drug laws encompasses 2C-I, 2C-E, DOI, ephedrine, and pseudoephedrine, classifying them as Schedule 3 compounds.[Note: Please note that drug laws may evolve, and it’s important to verify current regulations.
Portugal
Portugal has decriminalized the possession of recreational drugs in quantities not exceeding a ten-day supply. However, production and distribution (buying/selling) remain criminal offences.
Sweden
Sweden, through the health ministry of Sveriges riksdag, Statens folkhälsoinstitut, classifies 2C-E as a “health hazard” under the Act on the Prohibition of Certain Goods Dangerous to Health (Lagen om förbud mot vissa hälsofarliga varor). This classification was enacted on October 1, 2004, and is listed as 2,5-dimetoxi-4-etylfenetylamin (2C-E), thereby making it illegal to sell or possess.
UK
In the United Kingdom, 2C-E is categorized as a Class A controlled substance. The UK has one of the most stringent stances in the EU regarding designer drugs. The Misuse of Drugs Act was amended in 2002 to encompass a “catch most” clause, which outlaws substances like 2C-E, along with compounds from the LSD (ergoline) and MDMA (phenethylamine) chemical families. This amendment was significantly inspired by the work of American biochemist Alexander Shulgin, who extensively researched and documented various phenethylamine compounds and tryptamine compounds, including 2C-E. The Shulgins aimed to protect public access to information about psychedelic compounds, a mission Alexander Shulgin emphasized repeatedly.
United States
As of July 9, 2012, the United States classified 2C-E as a Schedule I substance under the Food and Drug Administration Safety and Innovation Act of 2012, making its possession, distribution, and manufacture illegal.
FAQ
1. What is 2C-E?
2C-E is a psychedelic compound, specifically a member of the 2C family of phenethylamines. It is known for its hallucinogenic properties.
2. How is 2C-E typically consumed?
2C-E is often ingested orally, usually in the form of a pill or powder. Some individuals may also choose to insufflate (snort) it, although this is less common.
3. Who was the originator of 2C-E?
2C-E was first synthesized by Alexander Shulgin, a prominent chemist and psychopharmacologist. It is documented in his book “PiHKAL.”
4. What is the recommended dosage for 2C-E?
The appropriate dosage for 2C-E can vary widely among individuals. In Alexander Shulgin’s “PiHKAL,” he mentions that a 10-milligram dosage may result in significant effects, while a 30-milligram trial can be intensely challenging. It’s essential to exercise caution and start with lower doses when experimenting with this substance.
5. What are the typical effects of 2C-E?
The effects of 2C-E are often described as “neutral” when compared to other psychedelic substances. Users may experience sensory distortions, changes in perception, and altered states of consciousness. The duration of effects can range from six to ten hours.
6. Is 2C-E legal?
The legal status of 2C-E varies by country and jurisdiction. Many nations have classified it as a controlled substance, making its production, distribution, and possession illegal. It’s important to understand and adhere to local laws and regulations.
7. What are the potential risks and side effects of 2C-E?
Adverse effects can include tachycardia (rapid heartbeat), hypertension (high blood pressure), agitation, delirium, and hallucinations. Overdoses can have severe consequences, so responsible use and caution are crucial.
8. Can 2C-E be used for therapeutic purposes?
Research on the therapeutic potential of 2C-E is limited, and it is not approved for medical or therapeutic use. Those interested in psychedelic therapy may explore legal and regulated options with substances like psilocybin or MDMA.
9. What is the legal history of 2C-E?
The legal status of 2C-E has evolved, with many countries classifying it as a controlled substance. Stay informed about the specific laws in your area.
References
- Alexander Shulgin and Ann Shulgin authored “PiHKAL: A Chemical Love Story,” a book published in September 1991. This publication is a valuable resource for understanding various psychoactive compounds. It’s available from Transform Press and is identified by ISBN 0-9630096-0-5. The OCLC number is 25627628.
- “2C-E in PiHKAL” is a notable entry in Alexander Shulgin’s book, “PiHKAL,” providing insights into the properties and effects of 2C-E.
- Alexander T. Shulgin, Tania Manning, Paul F. Daley published “The Shulgin Index: Volume 1” in 2011. This comprehensive volume serves as a reference for an array of chemical compounds, including 2C-E. The ISBN is 978-0-9630096-3-0.
- A case series regarding symptomatic patients, including one fatality, following exposure to 2C-E was reported by Topeff JM, Ellsworth H, Willhite LA, Bangh SA, Edwards EM, and Cole JB in 2011. Their findings can be found in the “Clin. Toxicol.” journal, volume 49, page 526.
- In March 2011, Sherisse Pham reported a case of a man arrested in a mass drug overdose incident involving 2C-E. The incident resulted in the tragic death of one teen and the hospitalization of ten individuals. This report was covered by ABC World News.
- Sacks J, Ray MJ, Williams S, and Opatowsky MJ documented a case of fatal toxic leukoencephalopathy stemming from an overdose of the designer drug 2C-E, often referred to as “Europa.” Their findings were published in the “Baylor University Medical Center Proceedings” in 2012 (volume 25, pages 374–376).
- In Queensland, Australia, 2C-E was included in the ‘Dangerous Drugs’ list of the ‘Drugs Misuse Act 1986’ by the ‘Drugs Misuse Amendment Act 2008,’ making its production, supply, and possession illegal. This is specified in the Queensland Legislation.
- Canada, as of May 4, 2016, introduced regulatory amendments under “Part J — 2C-phenethylamines” in the Food and Drug Regulations. These changes impacted the legal status of 2C-E in Canada.
- In China, 2C-E was designated as a controlled substance in October 2015 by the China Food and Drug Administration, rendering its production, supply, and possession illegal.
- Denmark added 2C-E to the list of Schedule B controlled substances, affecting its legal status within the country.
- Portugal decriminalized the possession of recreational drugs in quantities not exceeding a ten-day supply. However, the production and distribution (buying/selling) of such substances remain criminal offenses.
- Sweden, through the health ministry of Sveriges riksdag, Statens folkhälsoinstitut, classified 2C-E as a “health hazard” under the Act on the Prohibition of Certain Goods Dangerous to Health (Lagen om förbud mot vissa hälsofarliga varor) on October 1, 2004. It is listed as “2,5-dimetoxi-4-etylfenetylamin (2C-E),” thereby making its sale or possession illegal.
- In the United Kingdom, 2C-E is classified as a Class A controlled substance. The UK has stringent laws regarding designer drugs, partly influenced by the work of Alexander Shulgin, a renowned biochemist.
- In the United States, 2C-E was categorized as a Schedule I substance under the Food and Drug Administration Safety and Innovation Act of 2012, effective from July 9, 2012. This classification makes possession, distribution, and manufacture illegal.