Beautiful Plants For Your Interior
Summary
3-Hydroxyphencyclidine, known as 3-HO-PCP, is a dissociative substance belonging to the arylcyclohexylamine class and is closely related to phencyclidine (PCP). This compound has been available for purchase online as a designer drug.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 79787-43-2 79295-51-5 (hydrochloride) |
|---|---|
| PubChem CID | 133277 |
| ChemSpider | 117583 |
| UNII | V886QLHNA6 |
| CompTox Dashboard (EPA) | DTXSID20229918 |
| Chemical and physical data | |
| Formula | C17H25NO |
| Molar mass | 259.393 g·mol−1 |
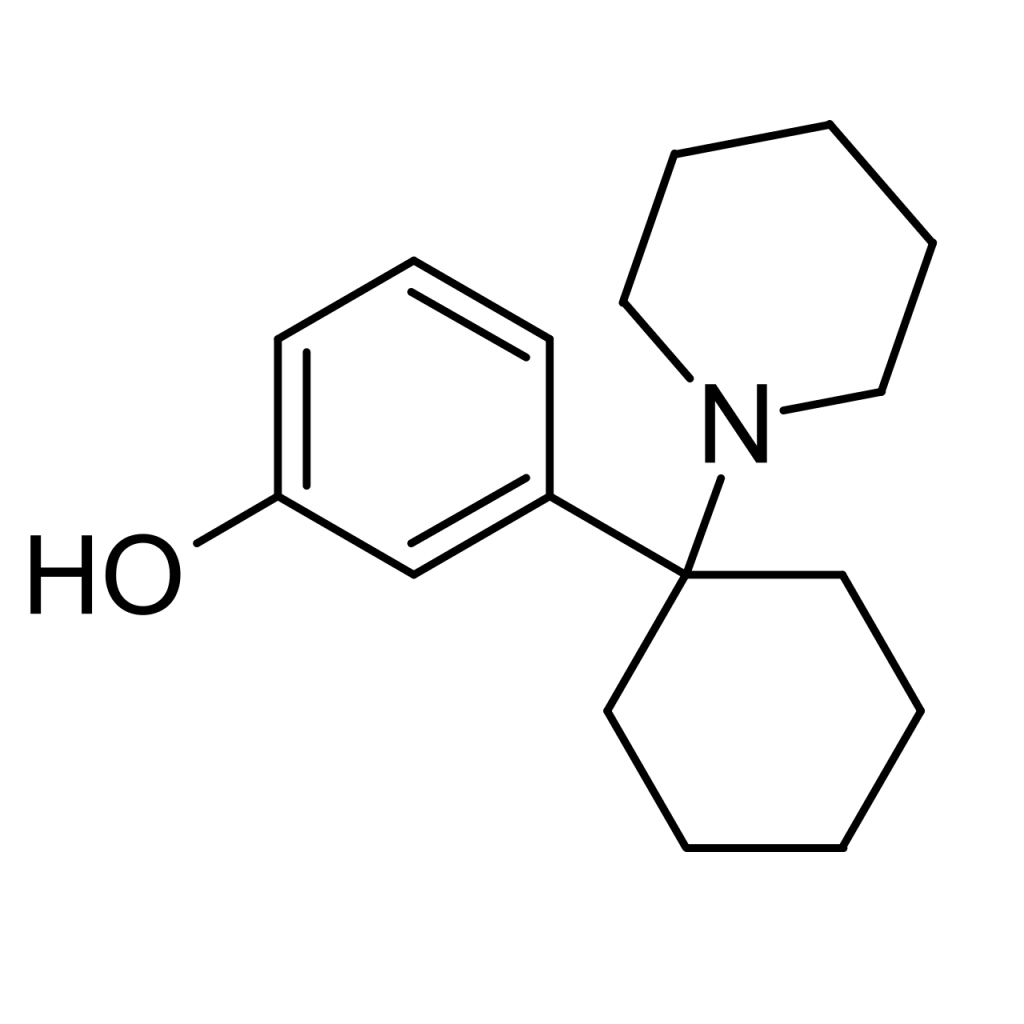
Pharmacology
3-Hydroxyphencyclidine, or 3-HO-PCP, exerts its pharmacological actions through high-affinity uncompetitive antagonism at the NMDA receptor, explicitly targeting the dizocilpine (MK-801) site with a Ki value of 30 nM. Notably, it exhibits significantly greater affinity for this site compared to PCP, with a Ki value of 250 nM, representing an eightfold difference.
Furthermore, in animal test subjects, 3-HO-PCP displays a substantial affinity for the μ-opioid receptor (MOR) with Ki values ranging from 39 to 60 nM. It also binds strongly to the κ-opioid receptor (KOR) with a Ki value of 140 nM and the sigma σ1 receptor, showing a Ki value of 42 nM and an IC50 value of 19 nM. However, it bears only a low affinity for the δ-opioid receptor with a Ki value of 2,300 nM. This distinct high affinity for opioid receptors sets 3-HO-PCP apart from other arylcyclohexylamines, particularly when compared to PCP, which has very low affinity for the MOR (Ki ranging from 11,000 to 26,000 nM, constituting a 282- to 433-fold difference) and other opioid receptors (Ki of 4,100 nM for the KOR and 73,000 nM for the DOR).
It’s worth noting that while there was initial speculation that 3-HO-PCP might be a metabolite of PCP in humans, there is currently no scientific evidence to support this hypothesis.
Chemistry
3-Hydroxyphencyclidine, commonly referred to as 3-HO-PCP, is categorized as an arylcyclohexylamine compound. It shares structural similarities with several compounds, including PCP, 3-MeO-PCP, 4-MeO-PCP, 3-MeO-PCMo, and more remotely, ketamine, methoxetamine, 3-MeO-PCE, methoxetamine, and dimetamine.
Society and culture
Legal status:
In the United Kingdom, on October 18, 2012, the Advisory Council on the Misuse of Drugs released a report concerning methoxetamine (MXE). The report concluded that the “harms of methoxetamine are commensurate with Class B of the Misuse of Drugs Act (1971),” even though the act does not categorize drugs based on harm. The report also recommended that all analogs of MXE, including 3-HO-PCP, should be classified as Class B drugs. This suggested a catch-all clause encompassing both known and unexplored arylcyclohexamines.
Furthermore, 3-HO-PCP is prohibited in Sweden and Switzerland.
FAQ
1. What is 3-Hydroxyphencyclidine (3-HO-PCP)?
- 3-Hydroxyphencyclidine, also known as 3-HO-PCP, is a chemical compound belonging to the arylcyclohexylamine class, often used for its dissociative effects.
2. How is 3-HO-PCP typically used?
- 3-HO-PCP is primarily used for recreational purposes. It can be ingested orally, inhaled, or insufflated (snorted). The method of use may vary among individuals.
3. What are the effects of 3-HO-PCP?
- The effects of 3-HO-PCP are characterized by dissociation, altered perception of reality, and a sense of detachment from one’s surroundings.
4. Is 3-HO-PCP legal?
- The legal status of 3-HO-PCP varies by country and region. It’s essential to be aware of the laws in your specific area regarding the possession and use of this substance.
5. Is 3-HO-PCP safe to use?
- The safety of using 3-HO-PCP is a subject of concern, as its long-term effects and potential risks are not well-documented. Like many designer drugs, there can be health risks associated with use, including unknown purity and possible adverse reactions.
6. What are the risks and side effects of using 3-HO-PCP?
- Common side effects may include nausea, disorientation, hallucinations, and impaired motor skills. Overdosing can lead to more severe symptoms such as anxiety, confusion, and potentially life-threatening conditions.
7. Can 3-HO-PCP be addictive?
- There is a potential for psychological dependence with repeated use, but it is not considered highly physically addictive like some other substances.
8. How can I reduce the risks associated with 3-HO-PCP use?
- If you choose to use 3-HO-PCP, it’s essential to start with a low dose, be in a safe environment, and have someone you trust present. Avoid mixing it with other substances and be aware of its potential adverse effects.
9. Is there a safe or recommended dosage for 3-HO-PCP?
- The dosage can vary based on an individual’s tolerance and experience. Starting with a low dose and monitoring your body’s response is advisable.
10. Where can I find help or support for 3-HO-PCP-related issues?
- If you or someone you know is struggling with substance use or addiction, it’s crucial to seek professional help. Reach out to addiction treatment centers or healthcare providers for guidance and support.
Please note that this information is for informational purposes only, and the use of designer drugs like 3-HO-PCP carries risks and legal implications. Always prioritize your health and safety and be aware of the laws in your area.
References
- In 2014, Morris H and Wallach J authored a comprehensive review titled “From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs.” This informative review was published in Drug Testing and Analysis, Volume 6 (7–8). You can access it via DOI: 10.1002/dta.1620 and PMID: 24678061.
- In July 2020, Davidsen AB, Mardal M, Johansen SS, Dalsgaard PW, and Linnet K conducted research on the metabolism and detection of 3-HO-PCP in human samples. Their findings were published in Drug Testing and Analysis, Volume 12 (7) and can be accessed through DOI: 10.1002/dta.2807. The PMID for this study is 32311838, and it is also available on S2CID with reference number 216047397.
- Kamenka JM, Chiche B, Goudal R, Geneste P, Vignon J, Vincent JP, and Lazdunski M explored the “Chemical synthesis and molecular pharmacology of hydroxylated 1-(1-phenylcyclohexyl-piperidine derivatives” in April 1982. Their research was published in the Journal of Medicinal Chemistry, Volume 25 (4). You can find it with the PMID: 6279847 and DOI: 10.1021/jm00346a019.
- In June 1984, Johnson N, Itzhak Y, and Pasternak GW investigated the “Interaction of two phencyclidine opiate-like derivatives with 3H-opioid binding sites.” This study was published in the European Journal of Pharmacology, Volume 101 (3–4) and is accessible via DOI: 10.1016/0014-2999(84)90171-7, with the PMID: 6088255.
- In July 1981, Itzhak Y, Kalir A, and Sarne Y delved into “On the opioid nature of phencyclidine and its 3-hydroxy derivative.” This research was published in the European Journal of Pharmacology, Volume 73 (2–3) and can be found with DOI: 10.1016/0014-2999(81)90097-2 and PMID: 6273187.
- In January 1985, Itzhak Y characterized specific binding sites for 3H-N-allylnormetazocine in rat brain membranes. This study was published in Molecular Pharmacology, Volume 27 (1) with the PMID: 3965930.
- In 1987, Itzhak Y explored “[3H]PCP-3-OH and (+)[3H]SKF 10047 binding sites in rat brain membranes: evidence of multiplicity.” The research was published in the European Journal of Pharmacology, Volume 136 (2) with DOI: 10.1016/0014-2999(87)90715-1 and PMID: 3036548.
- Itzhak Y conducted research in 1988 regarding the “Pharmacological specificity of some psychotomimetic and antipsychotic agents for the sigma and PCP binding sites.” This work was published in Life Sciences, Volume 42 (7) with DOI: 10.1016/0024-3205(88)90646-7 and PMID: 2893238.
- Holsztynska EJ and Domino EF contributed to a study on the “Biotransformation of phencyclidine” in 1985, which was published in Drug Metabolism Reviews, Volume 16 (3). It can be accessed via DOI: 10.3109/03602538508991437 and PMID: 3914938.
- In 1986, Holsztynska EJ and Domino EF conducted research on the “Quantitation of phencyclidine, its metabolites, and derivatives by gas chromatography with nitrogen-phosphorus detection.” This study was published in the Journal of Analytical Toxicology, Volume 10 (3) and can be found with DOI: 10.1093/jat/10.3.107 and PMID: 3724069.
- The “(ACMD) Methoxetamine Report (2012)” was released by the UK Home Office on October 18, 2012. This report discusses various aspects related to methoxetamine and can be accessed online.
- In Sweden, 3-HO-PCP is classified as a banned substance.
- Switzerland also prohibits the use and distribution of 3-HO-PCP.