Beautiful Plants For Your Interior
- 1 Summary
- 2 Legal Status
- 3 FAQ
- 3.1 1. What is 3′,4′-Methylenedioxy-α-pyrrolidinobutiophenone (MDPBP)?
- 3.2 2. What are the effects of MDPBP?
- 3.3 3. Is MDPBP legal?
- 3.4 4. What are the health risks associated with MDPBP use?
- 3.5 5. Can MDPBP be detected in drug tests?
- 3.6 6. Are there any street names for MDPBP?
- 3.7 7. Is MDPBP used for any medical purposes?
- 3.8 8. What should I do if I suspect someone is using MDPBP?
- 4 References
Summary
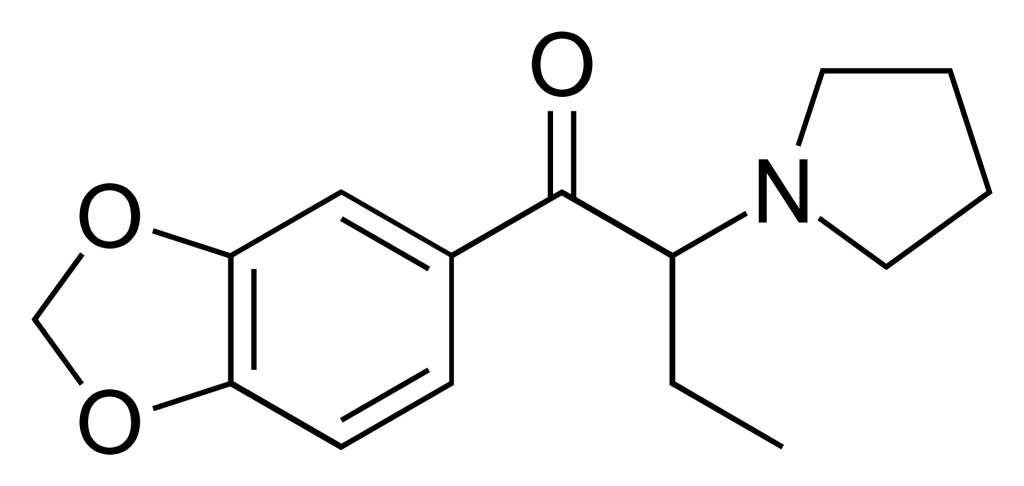
3′,4′-Methylenedioxy-α-pyrrolidinobutyrophenone (MDPBP) is a stimulant belonging to the cathinone class, initially developed in the 1960s. It has gained notoriety as a novel designer drug. MDPBP is sometimes marketed under the alias “NRG-1” in combination with other cathinone derivatives, including flephedrone, pentylone, MαPPP, and its higher homologue MDPV. As observed with other cathinones, MDPBP has demonstrated reinforcing effects in laboratory rat studies.
The primary metabolic processes are believed to involve demethylation, subsequent methylation of a hydroxy group, hydroxylation of the aromatic and side chains, oxidation of the pyrrolidine ring leading to the corresponding lactam, and ring opening to form the corresponding carboxylic acid. Demethylation is primarily catalyzed by the isoenzymes CYP2C19 and CYP2D6.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 784985-33-7 |
|---|---|
| PubChem CID | 71300672 |
| ChemSpider | 52084589 |
| UNII | 979L917V7T |
| Chemical and physical data | |
| Formula | C15H19NO3 |
| Molar mass | 261.321 g·mol−1 |
Legal Status
As of October 2015, Poland and China have classified MDPBP as a regulated substance.
FAQ
1. What is 3′,4′-Methylenedioxy-α-pyrrolidinobutiophenone (MDPBP)?
- 3′,4′-Methylenedioxy-α-pyrrolidinobutiophenone, often referred to as MDPBP, is a synthetic compound categorized as a stimulant of the cathinone class. It was initially developed in the 1960s and is known as a novel designer drug.
2. What are the effects of MDPBP?
- MDPBP is recognized for its stimulant effects, including increased alertness, energy, and euphoria. It may also lead to heightened sociability.
3. Is MDPBP legal?
- The legal status of MDPBP varies by country and region. In some areas, it is classified as a controlled substance and is illegal to manufacture, possess, or distribute without proper authorization. Be sure to check local regulations to determine its legal status.
4. What are the health risks associated with MDPBP use?
- Using MDPBP can carry various health risks, including an increased heart rate, anxiety, paranoia, and the potential for addiction. Long-term effects are not well understood due to limited research.
5. Can MDPBP be detected in drug tests?
- MDPBP can potentially be detected in drug tests, particularly in urine tests. However, its inclusion in standard drug screenings may vary. Specialized tests may be required for its detection.
6. Are there any street names for MDPBP?
- Street names for MDPBP may vary by region and include nicknames or slang terms commonly used by individuals in the illicit drug trade.
7. Is MDPBP used for any medical purposes?
- MDPBP is not approved for any medical use and is primarily encountered as a recreational or illicit substance.
8. What should I do if I suspect someone is using MDPBP?
- If you suspect someone is using MDPBP or any other illicit substance, it’s crucial to encourage them to seek professional help. Substance abuse can have severe health and social consequences, and early intervention is essential.
References
- DE 1545591, Koeppe H, Zeile K, Ludwig G, “Method for Producing α-Aminoketones with Heterocyclic Amino Group,” originally published on May 28, 1965, and granted on August 7, 1969, assigned to Boehringer Ingelheim.
- Kolanos R, Solis E, Sakloth F, De Felice LJ, Glennon RA (December 2013). “Breaking Down the Synthetic Cathinone Methylenedioxypyrovalerone (MDPV) and Investigating its Effects on the Human Dopamine Transporter.” Published in ACS Chemical Neuroscience. Volume 4, Issue 12, Pages 1524–1529. DOI: 10.1021/cn4001236. PMC 3867964. PMID 24116392.
- Westphal F, Junge T, Klein B, Fritschi G, Girreser U (June 2011). “Spectroscopic Characterization of 3,4-Methylenedioxypyrrolidinobutyrophenone: A New Designer Drug with α-Pyrrolidinophenone Structure.” Published in Forensic Science International. Volume 209, Issues 1–3, Pages 126–132. DOI: 10.1016/j.forsciint.2011.01.016. PMID 21316166.
- Wiergowski M, Woźniak MK, Kata M, Biziuk M (August 2016). “Determination of MDPBP in Postmortem Blood Samples by Gas Chromatography Coupled with Mass Spectrometry.” Published in Monatshefte für Chemie. Volume 147, Issue 8, Pages 1415–1421. DOI: 10.1007/s00706-016-1780-0. PMC 4971041. PMID 27546909.
- Brandt SD, Freeman S, Sumnall HR, Measham F, Cole J (September 2011). “Analysis of NRG ‘Legal Highs’ in the UK: Identification and Formation of Novel Cathinones.” Published in Drug Testing and Analysis. Volume 3, Issue 9, Pages 569–575. DOI: 10.1002/dta.204. PMID 21960541.
- Gannon BM, Galindo KI, Mesmin MP, Sulima A, Rice KC, Collins GT (May 2018). “Relative Reinforcing Effects of Second-Generation Synthetic Cathinones: Acquisition of Self-Administration and Fixed Ratio Dose-Response Curves in Rats.” Published in Neuropharmacology. Volume 134, Part A, Pages 28–35. DOI: 10.1016/j.neuropharm.2017.08.018. PMC 5809320. PMID 28811192.
- Meyer MR, Mauer S, Meyer GM, Dinger J, Klein B, Westphal F, Maurer HH (July–August 2014). “In Vivo and In Vitro Metabolism and the Detectability in Urine of 3′,4′-Methylenedioxy-α-Pyrrolidinobutyrophenone (MDPBP), a New Pyrrolidinophenone-Type Designer Drug.” Published in Drug Testing and Analysis. Volume 6, Issues 7–8, Pages 746–756. DOI: 10.1002/dta.1559. PMID 24124065.
- “关于印发《非药用类麻醉药品和精神药品列管办法》的通知” (in Chinese). China Food and Drug Administration. September 27, 2015. Archived from the original on October 1, 2015. Retrieved on October 1, 2015.