Beautiful Plants For Your Interior
Summary
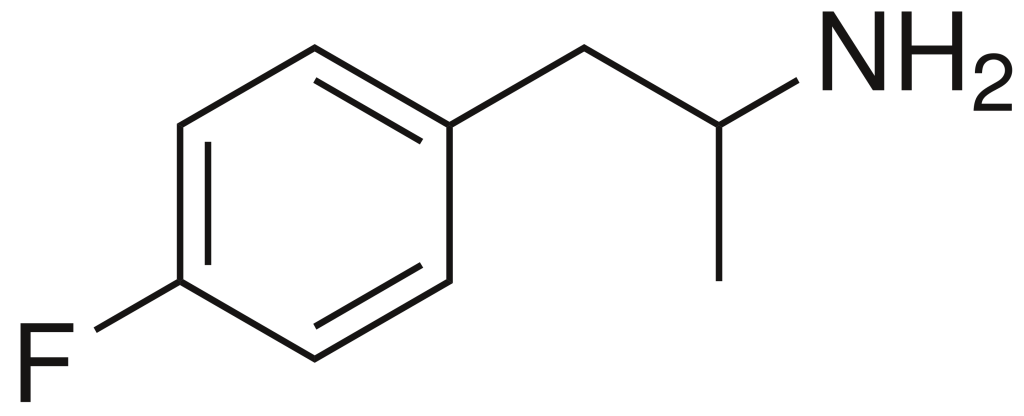
4-Fluoroamphetamine (4-FA), also recognized as 4-FMP, PAL-303, or “Flux,” and alternatively referred to as para-fluoroamphetamine (PFA), belongs to the realm of psychoactive research chemicals, falling within the phenethylamine and substituted amphetamine categories. It is characterized by its capacity to induce both stimulating and entactogenic effects. In recreational contexts, 4-FA is occasionally available alongside analogous compounds like 2-fluoroamphetamine and 4-fluoromethamphetamine.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 459-02-9 |
|---|---|
| PubChem CID | 9986 |
| ChemSpider | 9592 |
| UNII | S5744XYR1Z |
| CompTox Dashboard (EPA) | DTXSID90894758 |
| Chemical and physical data | |
| Formula | C9H12FN |
| Molar mass | 153.200 g·mol−1 |
Usage
4-FA gained popularity in the Netherlands, with the primary attraction being its specific effects, favored by 77% of users, rather than its legal status, which appealed to only 18% of the users. However, it’s important to note that 4-FA was declared illegal in May 2017.
Effects
The subjective experiences associated with 4-fluoroamphetamine encompass sensations of euphoria, which some liken to the effects of MDMA and amphetamine. Other effects include:
- Heightened energy levels (stimulation).
- An elevated mood.
- Feelings of warmth and empathy.
- Excessive talking.
- Bruxism (teeth grinding).
- A reduced appetite (anorexic).
The general progression of effects typically commences with primarily empathogenic results during the initial hours, gradually transitioning to increased stimulation over the next several hours.
Notably, 4-FA exhibits a more potent dopamine reuptake inhibition compared to compounds like 4-CA or 4-IA. Additionally, it tends to produce less hyperthermia when contrasted with similar substances such as PMA, 3-MTA, and 4-methylamphetamine.
Common acute side effects encompass nausea, headaches, an accelerated heart rate, and difficulty falling asleep (insomnia).
Chemistry
| Reagent | Reaction color |
|---|---|
| Marquis | No reaction |
| Mandelin | Pale Blue |
| Liebermann | Orange |
| Froehde | Faint purple/brown or no reaction. |
Pharmacology
4-Fluoroamphetamine, commonly known as 4-FA, operates as both a releasing agent and a reuptake inhibitor for dopamine, serotonin, and norepinephrine. This compound exhibits distinct values for its EC50 and IC50, emphasizing its varied interactions with these neurotransmitters.
When considering the metabolic destiny of 4-FA, it’s worth noting that the C-F bond at the 4-position on the phenyl ring appears to resist deactivation within the liver, attributed to cytochrome P450 oxidase.
Neurotoxicity
In contrast to its analogs 4-CA and 4-BA, 4-FA does not induce long-lasting depletion of brain serotonin. This phenomenon is believed to result from the unique metabolic pathways of the fluoro compound, setting it apart from other halo amphetamines.
Surprisingly, the level of neurotoxicity does not escalate across para-halogenated amphetamine derivatives despite serotonin-releasing potency following a discernible trend. For instance, 4-iodoamphetamine is less toxic than 4-chloroamphetamine, suggesting that para-substitution doesn’t inherently increase serotonergic neurotoxicity. Notably, some exceptions to this rule include 4-MTA, which is para-substituted and non-neurotoxic.
Toxicology
The LD50 (lethal dose for 50% of subjects) for 4-FA, when administered intraperitoneally in mice, is 46 mg/kg.
Regrettably, there have been reported cases of fatalities associated with 4-FA. In one instance, 4-FA mixed with 25C-NBOMe in a capsule was linked to three deaths in Melbourne in 2017.
Legal Status
As of October 2015, 4-FA has been classified as a controlled substance in China. Its legal status also denotes that it is banned in the Czech Republic, and as of May 25, 2017, it has been designated a controlled substance in the Netherlands. Furthermore, 4-FA is regulated in numerous countries, including Australia, Belgium, the UK, Germany, Israel, Slovakia, Bulgaria, Chile, Brazil, Canada, Croatia, Sweden, New Zealand, and France.
FAQ
1. What is 4-Fluoroamphetamine (4-FA)?
4-Fluoroamphetamine, commonly known as 4-FA, is a chemical compound falling within the phenethylamine and substituted amphetamine classes. It is recognized for its psychoactive properties, inducing stimulant and entactogenic effects.
2. What are the shared names or aliases for 4-FA?
4-FA is sometimes referred to as 4-FMP, PAL-303, or “Flux.” It is also known by its systematic name, para-fluoroamphetamine (PFA).
3. How does 4-FA affect the brain and body?
4-FA acts as both a releasing agent and a reuptake inhibitor for dopamine, serotonin, and norepinephrine. Its effects include sensations of euphoria, increased energy, mood elevation, empathy, excessive talking, bruxism (teeth grinding), and reduced appetite.
4. Is 4-FA similar to MDMA (Ecstasy) or amphetamines?
4-FA’s effects may be similar to both MDMA and amphetamines, particularly in terms of mood enhancement and increased energy.
5. What is the legal status of 4-FA in different countries?
The legal status of 4-FA varies by country. For example, it has been classified as a controlled substance in China and is banned in the Czech Republic and the Netherlands. It is also regulated in many other countries, including Australia, Belgium, the UK, Germany, and more.
6. Are there risks or side effects associated with 4-FA use?
Common side effects of 4-FA may include nausea, headaches, an increased heart rate, and difficulty falling asleep (insomnia). It is essential to be aware of the potential risks and follow safety guidelines when considering its use.
7. Is 4-FA associated with neurotoxicity or long-term brain effects?
Unlike some of its analogs, 4-FA does not cause long-lasting depletion of brain serotonin. Its metabolic pathways differ from other similar compounds, potentially contributing to this difference.
8. What is the LD50 of 4-FA?
The LD50 (lethal dose for 50% of subjects) for 4-FA, when administered intraperitoneally in mice, is 46 mg/kg.
9. Is 4-FA safe for recreational use?
The safety of 4-FA for recreational use is a subject of ongoing research and debate. It is essential to approach its use with caution, be aware of legal regulations, and prioritize safety.
10. Where can I find more information about 4-FA?
For more in-depth and up-to-date information about 4-FA, consider consulting scientific literature, government regulations, and reputable sources related to pharmacology and substance use. Always prioritize safety and adherence to relevant laws and regulations.
References
- Rösner P, Quednow B, Girreser U, Junge T (March 2005). “Isomeric fluoro-methoxy-phenylalkylamines: a new series of controlled-substance analogues (designer drugs)”. This study delves into the emergence of controlled-substance analogs, such as designer drugs, specifically focusing on isomeric fluoro-methoxy-phenylalkylamines. It offers valuable insights into this class of substances.
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K (March 2007). “The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain”. This research explores how psychoactive drugs impact monoamine neurotransmission in the brains of rats. It sheds light on the neural effects of these substances.
- Linsen F, Koning RP, van Laar M, Niesink RJ, Koeter MW, Brunt TM (July 2015). “4-Fluoroamphetamine in the Netherlands: more than a one-night stand”. This study investigates the prevalence and use patterns of 4-Fluoroamphetamine (4-FA) in the Netherlands, providing a comprehensive understanding of its role in the recreational drug scene.
- “Het is nu officieel: de partydrug 4-FA is verboden”. nos.nl. 25 May 2017. Archived from the original on 24 August 2017. This news report highlights the official prohibition of the party drug 4-FA in May 2017 in the Netherlands, indicating changes in its legal status.
- Marona-Lewicka D, Rhee GS, Sprague JE, Nichols DE (December 1995). “Psychostimulant-like effects of p-fluoroamphetamine in the rat”. This research delves into the psychostimulant effects of p-fluoroamphetamine in rats, shedding light on its potential psychoactive properties.
- “4-FA reaction colour results with liebermann and froehde reagent test kits”. Reagent Tests UK. 3 January 2016. Archived from the original on 14 February 2016. This source provides insights into the use of reagent test kits to detect 4-FA and its reaction color results.
- Uchiyama N, Kawamura M, Kamakura H, Kikura-Hanajiri R, Goda Y (June 2008). “[Analytical data of designated substances (Shitei-Yakubutsu) controlled by the Pharmaceutical Affairs Law in Japan, part II: Color test and TLC]”. This research offers analytical data regarding substances controlled by the Pharmaceutical Affairs Law in Japan, including 4-FA.
- Toennes SW, Schneider D, Pogoda W, Paulke A, Wunder C, Theunissen EL, et al. (July 2019). “Pharmacokinetic properties of 4-fluoroamphetamine in serum and oral fluid after oral ingestion”. This study examines the pharmacokinetic properties of 4-FA in serum and oral fluid following oral ingestion, providing essential information for understanding its metabolic pathways.
- Fisher MB, Henne KR, Boer J (January 2006). “The complexities inherent in attempts to decrease drug clearance by blocking sites of CYP-mediated metabolism”. This article discusses the complexities associated with efforts to reduce drug clearance by targeting sites of CYP-mediated metabolism.
- Harvey JA (June 1978). “Neurotoxic action of halogenated amphetamines”. This research explores the neurotoxic effects of halogenated amphetamines, offering insights into their potential adverse neurological impacts.
- Fuller RW, Baker JC, Perry KW, Molloy BB (October 1975). “Comparison of 4-chloro-, 4-bromo- and 4-fluoroamphetamine in rats: drug levels in brain and effects on brain serotonin metabolism”. This study compares the effects of 4-chloro-, 4-bromo-, and 4-fluoroamphetamine in rats, particularly focusing on their levels in the brain and their impact on brain serotonin metabolism.
- Nichols DE, Johnson MP, Oberlender R (January 1991). “5-Iodo-2-aminoindan, a nonneurotoxic analogue of p-iodoamphetamine”. This study discusses 5-iodo-2-aminoindan as a nonneurotoxic analogue of p-iodoamphetamine, highlighting its potential as a less harmful alternative.
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, et al. (June 2005). “Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration”. This research focuses on the development of a biogenic amine releaser designed to have a low abuse potential and its efficacy in reducing cocaine self-administration.
- Blanckaert P, van Amsterdam J, Brunt T, van den Berg J, Van Durme F, Maudens K, van Bussel J (September 2013). “4-Methyl-amphetamine: a health threat for recreational amphetamine users”. This study examines the potential health risks associated with 4-methyl-amphetamine, particularly among recreational amphetamine users.
- Huang X, Marona-Lewicka D, Nichols DE (December 1992). “p-methylthioamphetamine is a potent new non-neurotoxic serotonin-releasing agent”. This research investigates p-methylthioamphetamine as a potent non-neurotoxic serotonin-releasing agent, providing insights into its potential pharmacological properties.
- Li Q, Murakami I, Stall S, Levy AD, Brownfield MS, Nichols DE, Van de Kar LD (December 1996). “Neuroendocrine pharmacology of three serotonin releasers: 1-(1,3-benzodioxol-5-yl)-2-(methylamino)butane (MBDB), 5-methoxy-6-methyl-2-aminoindan (MMAi) and p-methylthioamphetamine (MTA)”. This research explores the neuroendocrine pharmacology of three serotonin releasers, including 1-(1,3-benzodioxol-5-yl)-2-(methylamino)butane (MBDB), 5-methoxy-6-methyl-2-aminoindan (MMAi), and p-methylthioamphetamine (MTA).
- Murphy J, Flynn JJ, Cannon DM, Guiry PJ, McCormack P, Baird AW, et al. (May 2002). “In vitro neuronal and vascular responses to 5-hydroxytryptamine: modulation by 4-methylthioamphetamine, 4-methylthiomethamphetamine, and 3,4-methylenedioxymethamphetamine”. This research explores the in vitro responses of neurons and vascular systems to 5-hydroxytryptamine, particularly how they are modulated by substances such as 4-methylthioamphetamine, 4-methylthiomethamphetamine, and 3,4-methylenedioxymethamphetamine.
- Costa E, Garattini S (1970). Amphetamines and Related Compounds. This book provides insights into amphetamines and related compounds, offering a comprehensive overview of their properties and effects.
- “News: March 2017 – Australia: ‘Ecstasy’ capsules containing NPS are related to several deaths and severe intoxications in Melbourne.” This news article reports on the association between “Ecstasy” capsules containing new psychoactive substances (NPS) and multiple deaths and severe intoxications in Melbourne, Australia.
- “关于印发《非药用类麻醉药品和精神药品列管办法》的通知” (in Chinese). China Food and Drug Administration. This official Chinese document outlines regulations concerning the control and classification of non-medical psychoactive substances, including 4-FA.
- “Látky, o které byl doplněn seznam č. 4 psychotropních látek (příloha č. 4 k nařízení vlády č. 463/2013 Sb.)” (PDF) (in Czech). Ministerstvo zdravotnictví. This document in the Czech language provides information about substances that have been added to the list of psychotropic substances, including 4-FA, based on government regulations.
- Van der Velden L (25 May 2017). “Vanaf vandaag is partydrug 4-FA officieel verboden – maar of dat helpt?” (in Dutch). de Volkskrant. This Dutch news report discusses the official prohibition of the party drug 4-FA in the Netherlands and its potential impact.