Beautiful Plants For Your Interior
Summary
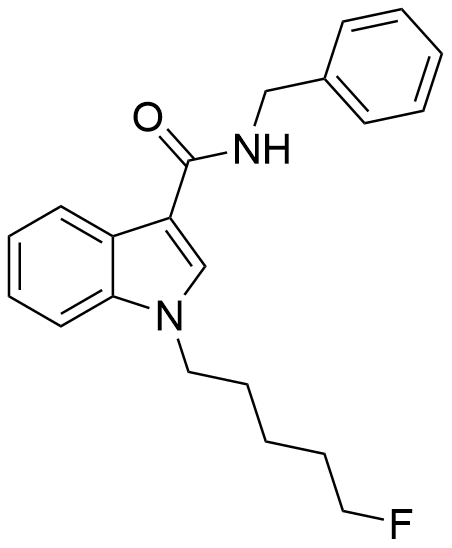
5F-SDB-006 is a substance known to function as a potent agonist for cannabinoid receptors. It exhibits an EC50 of 50 nM for human CB1 receptors and 123 nM for human CB2 receptors. This compound was first identified during investigations into the closely related substance APICA, which had been illicitly marketed under the name “2NE1”. Notably, 5F-SDB-006 is the terminally fluorinated counterpart of SDB-006, just as STS-135 serves as the terminally fluorinated analogue of APICA. Additionally, similar to the related compound APINACA, metabolic hydrolysis of the amide group within 5F-SDB-006 may release benzylamine.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1776086-02-2 |
|---|---|
| PubChem CID | 91936847 |
| ChemSpider | 30646773 |
| UNII | 4UP4SXX8BW |
| Chemical and physical data | |
| Formula | C21H23FN2O |
| Molar mass | 338.426 g·mol−1 |
FAQ
1. What is 5F-SDB-006?
- 5F-SDB-006 is a synthetic compound that acts as a potent agonist for cannabinoid receptors, specifically CB1 and CB2 receptors. It has gained attention due to its receptor affinity and potential effects.
2. What are the EC50 values for 5F-SDB-006’s interaction with cannabinoid receptors?
- The EC50 (half-maximal effective concentration) for 5F-SDB-006 is approximately 50 nM for human CB1 receptors and 123 nM for human CB2 receptors. These values indicate the compound’s potency in activating these receptors.
3. How was 5F-SDB-006 discovered?
- 5F-SDB-006 was identified during research into a related compound called APICA, which had been illegally distributed under the street name “2NE1.” The exploration of these substances led to the discovery of 5F-SDB-006.
4. What is the relationship between 5F-SDB-006 and SDB-006?
- 5F-SDB-006 is the terminally fluorinated analogue of SDB-006. This means that it shares a structural similarity with SDB-006, with the addition of a fluorine atom at a specific position.
5. Is there a parallel between 5F-SDB-006 and APICA regarding structural analogues?
- Yes, just as 5F-SDB-006 is related to SDB-006, the compound STS-135 is the terminally fluorinated analogue of APICA. This demonstrates a common trend in the structural modification of synthetic cannabinoids.
6. Are there concerns about the metabolic breakdown of 5F-SDB-006?
- Yes, similar to the related compound APINACA, there are suspicions that the metabolic hydrolysis of the amide group within 5F-SDB-006 may release benzylamine. This has raised concerns about potential health implications.
7. Is 5F-SDB-006 legally available or used for any specific purposes?
- The legal status of 5F-SDB-006 varies by country and jurisdiction. It is essential to be aware of the specific laws and regulations in your area regarding the use, possession, and distribution of this compound.
8. Where can I find more information about 5F-SDB-006 and its effects?
- For comprehensive information about 5F-SDB-006, its chemical properties, potential health risks, and legal status, consult reputable sources such as government health agencies, medical professionals, and substance abuse organizations.
References
- In an August 2015 study led by Banister SD and his team, the influence of bioisosteric fluorine in synthetic cannabinoid designer drugs was explored. The study focused on a range of compounds, including JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135. The investigation delved into the effects of introducing fluorine atoms into these synthetic cannabinoids. This research, published in ACS Chemical Neuroscience (Volume 6, Issue 8), can be accessed via doi:10.1021/acschemneuro.5b00107, and it is documented under PMID 25921407.
- In a study conducted by Banister SD, Wilkinson SM, and their team in July 2013, the synthesis and pharmacological evaluation of adamantane-derived indoles were examined. These compounds were classified as cannabimimetic drugs of abuse, and the study encompassed various aspects of their properties. Published in ACS Chemical Neuroscience (Volume 4, Issue 7), the research can be referenced through doi:10.1021/cn400035r. Additionally, the study is available with PMC 3715837 and PMID 23551277.