The online market for research chemicals has grown in popularity over the years, with many vendors offering a wide range of substances, including 5-MeO-MiPT. However, the proliferation of these sellers raises significant concerns regarding the quality, safety, and ethics of purchasing such substances.
Firstly, when considering where to buy 5-MeO-MiPT or any research chemical, it’s imperative to scrutinize the seller’s credibility. Many online vendors claiming to offer 5-MeO-MiPT for sale lack transparency and regulation. This raises red flags about the authenticity and purity of the product, which can pose serious health risks to consumers.
Furthermore, the sale of designer drugs like 5-MeO-MiPT often exists in a legal gray area, and some vendors may exploit this ambiguity to operate without adhering to safety standards or ethical guidelines. This lack of oversight can result in contaminated or mislabeled products, putting buyers at risk.
Additionally, ethical concerns surround the sale of research chemicals. Many vendors market these substances as “not for human consumption” to circumvent legal issues, yet they know that they are often used recreationally. This raises questions about the responsibility and accountability of these sellers.
To protect your health and well-being, it is essential to exercise caution when considering purchasing 5-MeO-MiPT or any research chemical online. Research thoroughly, seek out reputable vendors with a history of transparency and quality control, and prioritize your safety. Always keep in mind the potential legal consequences and ethical implications associated with the purchase and use of these substances.
- 1 Summary
- 2 Chemistry
- 3 Pharmacology
- 4 Subjective effects
- 5 Reagent results
- 6 Toxicity
- 7 Legal status
- 8 FAQ
- 8.1 1. What is 5-MeO-MiPT?
- 8.2 2. What are the effects of 5-MeO-MiPT?
- 8.3 3. Is 5-MeO-MiPT legal?
- 8.4 4. What are the potential risks of using 5-MeO-MiPT?
- 8.5 5. Is 5-MeO-MiPT addictive?
- 8.6 6. What is the recommended dosage of 5-MeO-MiPT?
- 8.7 7. How long do the effects of 5-MeO-MiPT last?
- 8.8 8. Can 5-MeO-MiPT be detected in drug tests?
- 8.9 9. How can I reduce the risks associated with using 5-MeO-MiPT?
- 9 References
Summary
5-Methoxy-N-methyl-N-isopropyltryptamine, commonly called 5-MeO-MiPT or moxy, belongs to the lesser-known class of tryptamine psychedelics. This compound resembles other tryptamines, such as 5-MeO-DMT and 5-MeO-DiPT, and exerts its psychoactive effects by interacting with serotonin receptors within the brain.
The synthesis and pharmacological characteristics of 5-MeO-MiPT were initially documented in 1985 by David Repke and Alexander Shulgin. Shulgin’s comprehensive exploration of this substance can be found in his book, “TiHKAL” (Tryptamines I Have Known and Loved), where its effects on humans were documented.
According to anecdotal accounts, 5-MeO-MiPT’s effects are primarily characterized as highly stimulating and mildly entactogenic, with a noteworthy absence of typical psychedelic visual distortions. Many users describe pronounced physical and tactile sensations that often intensify libido and heighten sexual pleasure. However, it is essential to note that common to high doses of this substance may be accompanied by an uncomfortable “body load” marked by muscle tension and nausea.
The knowledge regarding the pharmacology, metabolism, and potential toxicity of 5-MeO-MiPT remains relatively limited, and its history of human use is relatively short. It has been made available online under the guise of a research chemical. Given these uncertainties, it is strongly recommended to employ harm reduction practices when considering the use of this substance. The lack of comprehensive scientific research and regulatory oversight underscores the importance of cautious and responsible experimentation with 5-MeO-MiPT to minimize potential risks to one’s health and well-being.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 96096-55-8 |
|---|---|
| PubChem CID | 2763156 |
| ChemSpider | 2043845 |
| UNII | L0P1807EUY |
| ChEMBL | ChEMBL172139 |
| CompTox Dashboard (EPA) | DTXSID90242114 |
| ECHA InfoCard | 100.223.426 |
| Chemical and physical data | |
| Formula | C15H22N2O |
| Molar mass | 246.354 g·mol−1 |
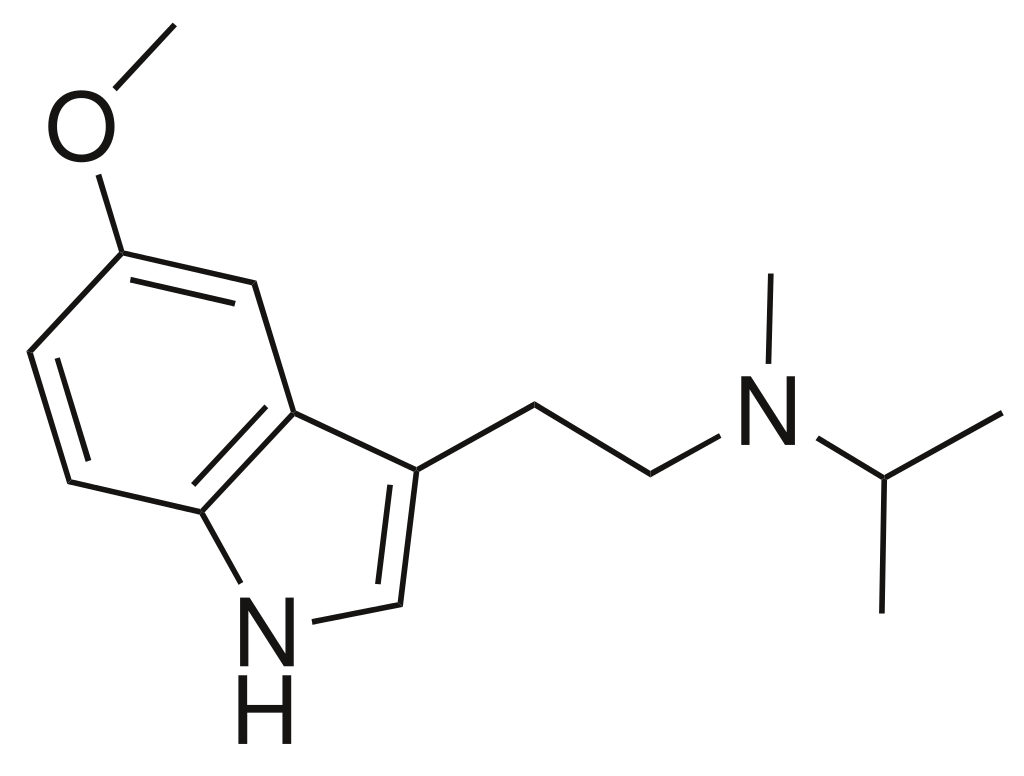
Chemistry
5-MeO-MiPT, scientifically known as 5-methoxy-N-methyl-N-isopropyltryptamine, falls within the synthetic indole alkaloid category, specifically within the tryptamine class. Tryptamines share a common structural foundation: a bicyclic indole heterocycle connected at R3 to an amino group through an ethyl side chain. In the case of 5-MeO-MiPT, this compound features a substitution at R5 of its indole heterocycle with a methoxy (MeO) functional group (CH3O−). Additionally, it possesses a methyl group and an isopropyl chain linked to the terminal amine RN of its tryptamine framework (MiPT).
It’s worth noting that 5-MeO-MiPT represents the N-substituted isopropyl counterpart of 5-MeO-DMT within this chemical class.
Pharmacology
The psychedelic effects of 5-MeO-MiPT are thought to stem primarily from its partial agonist activity at the 5-HT2A receptor, as supported by scientific literature. Additionally, there have been speculative theories regarding other potential mechanisms of action, such as inhibiting monoamine oxidase (MAO) enzymes in the stomach. However, this remains unproven through scientific investigation.
While 5-MeO-MiPT exhibits its strongest binding affinity to 5-HT1A receptors, it also demonstrates a notable affinity for the serotonin transporter (SERT) and the norepinephrine transporter (NET), effectively functioning as a moderately potent serotonin-norepinephrine reuptake inhibitor (SNRI). These multifaceted mechanisms of action may account for the numerous anecdotal reports describing antidepressant and anxiolytic effects associated with modest doses of this compound.
To illustrate, common pharmaceuticals used in treating depression, such as venlafaxine, belong to the class of SNRIs, which similarly impact serotonin and norepinephrine reuptake. Likewise, buspirone primarily targets 5-HT1A receptors and is a prescription medication mainly employed for managing anxiety. These parallels in pharmacological activity provide a potential framework for understanding the reported mood-enhancing and anxiety-reducing properties of 5-MeO-MiPT when administered at lower doses.
Subjective effects
5-MeO-MiPT can be administered through oral ingestion or smoking, each offering distinct experiential characteristics. When taken orally, users frequently report heightened visual and sensory effects. This experience unfolds in two stages: the initial phase is marked by stimulation and entactogenic qualities. At the same time, the latter resembles the more traditional effects of tryptamine psychedelics like psilocybin mushrooms or LSD. On the other hand, when smoked, the compound accentuates its physically and cognitively stimulating aspects.
However, it’s important to emphasize a disclaimer regarding the effects of 5-MeO-MiPT. These effects are sourced from the Subjective Effect Index (SEI), derived from anecdotal user accounts and analyses by contributors to PsychonautWiki. Therefore, they should be approached with a degree of skepticism.
Furthermore, it’s worth noting that these effects may not manifest predictably or consistently, and higher doses are more likely to produce the full spectrum of products. At the same time, increased amounts also raise the likelihood of adverse effects, including addiction, severe injury, or even fatality ☠.
Physical:
- Stimulation: At doses below 10 to 15mg, 5-MeO-MiPT generates motivation akin to LSD.
- Spontaneous bodily sensations: Users often describe a pleasurable, warm, enveloping body high accompanied by sporadic, unpredictable, cold, sharp tingling sensations.
- Nausea: Nausea, sometimes leading to vomiting, can occur, usually fading after the initial phase.
- **Bodily pressures, abnormal heartbeat, increased heart rate, blood pressure, headaches, dehydration, stomach bloating, pupil dilation, vasoconstriction, and diarrhea may also manifest.
Visual:
- Enhancements may include increased color perception, pattern recognition, and visual acuity.
- Distortions: Visual distortions like drifting, tracers, afterimages, symmetrical texture repetition, color shifting, and scenery slicing can occur.
- Geometry: The visual geometry resembles Psilocin, 4-AcO-DMT, or ayahuasca, characterized by complexity, abstractness, organic textures, bright multicolored schemes, glossy shading, and more.
Hallucinatory States:
- 5-MeO-MiPT induces a range of high-level hallucinatory experiences, often more consistent and reproducible than other psychedelics.
- Transformations and internal hallucinations (including autonomous entities, settings, sceneries, landscapes, and perspective alterations) are common and frequently influenced by personal, spiritual, science-fiction, fantasy, surreal, nonsensical, or transcendental themes.
Cognitive:
- At lower to moderate doses, users find 5-MeO-MiPT to be insightful, moderately relaxing, and sometimes stimulating. It produces typical psychedelic cognitive effects that intensify with dosage.
- Higher doses, however, tend to be primarily sedating and impairing, often leading to depersonalization without accompanying insight.
- Cognitive effects may include analysis enhancement, enhanced empathy, affection, and sociability (especially in social contexts), depersonalization, conceptual thinking, increased music appreciation, heightened emotions, increased libido, memory suppression, novelty enhancement, personal bias suppression, accelerated thought processes, thought connectivity, time distortion, and promoted wakefulness.
Auditory:
- Auditory effects may involve enhancements, distortions, and hallucinations.
Reagent results
Exposing compounds to the reagents gives a colour change which is indicative of the compound under test. The following test results are from ProTestKit.
| 5-MeO-MiPT | Marquis | Mecke | Mandelin | Liebermann | Ehrlich | Hofmann | Simon’s |
|---|---|---|---|---|---|---|---|
| Freebase | Orange – brown | Orange red | Deep greenish brown | Unknown | Purple | No reaction | No reaction |
| HCl | Orange – brown | Red – brown | Greenish brown | Brown | Violet – purple | Green | Unknown |
Toxicity
Very little is understood about the potential long-term consequences of 5-MeO-MiPT, and the precise toxic threshold remains elusive. This is primarily due to its status as a research chemical with a scant history of human usage.
An initial study involving mice indicated that a typical 5-MeO-MiPT dose (0.27 mg/kg) did not produce measurable toxic effects. However, when doses exceeding the normal range (2.7 mg/kg) were administered, cell toxicity emerged, leading to programmed cell death in the brain, liver, and kidneys. Whether similar damage occurs in other bodily tissues and the exact mechanisms involved remains unclear.
Anecdotal reports from individuals within the community who have experimented with 5-MeO-MiPT suggest that there are generally no adverse health effects associated with using the substance at low to moderate doses, provided it is used sparingly. Nonetheless, it is crucial to remember that no guarantees can be made, and independent research should always precede the consumption of any substance or combination of importance to ensure safety.
Harm reduction practices are highly recommended when engaging with this substance.
Tolerance and Addiction Potential:
5-MeO-MiPT is not considered habit-forming, and the desire to use it may decrease with continued use. Users often self-regulate their consumption.
Tolerance to 5-MeO-MiPT’s effects develops almost immediately after ingestion. Subsequently, it takes approximately three days for the patience to diminish by half and about seven days to return to baseline (assuming no further consumption). Notably, 5-MeO-MiPT creates cross-tolerance with all psychedelics, meaning that its use reduces the effectiveness of other psychedelics.
Dangerous Interactions:
It is crucial to exercise caution when combining substances, as many psychoactive substances, while safe when used individually, can become hazardous or even life-threatening when combined with others. Here are some known dangerous interactions:
- 2C-T-X: Both classes of compounds can have unpredictable effects when taken together.
- 2C-X: 5-MeO psychedelics may interact unpredictably and potentiate other psychedelics.
- Cannabis: Combining with cannabis may elevate the risk of adverse psychological effects such as anxiety, paranoia, and psychosis.
- DOx: Interaction with 5-MeO tryptamines can be unpredictable and may increase the risk of unpleasant physical side effects.
- MDMA: Some 5-MeO tryptamines can have unpredictable interactions with MDMA and should be used cautiously.
- Mescaline: 5-MeO tryptamines can interact unpredictably with mescaline.
- NBOMe: Combining 5-MeO tryptamines with NBOMes is best avoided, as both classes of compounds can have unpredictable interactions.
- Amphetamines: Combining with stimulants increases the risk of unpleasant thought loops and is generally unnecessary due to the stimulating effects of psychedelics.
- Cocaine: Similar to amphetamines, this combination increases the chance of unpleasant thought loops and is typically unnecessary.
- DXM: More information is needed about this combination.
- Tramadol: It elevates the risk of serotonin syndrome when combined.
- aMT: Increases the risk of serotonin syndrome.
- MAOIs: Interaction with MAOIs increases the risk of serotonin syndrome.
- PCP: There is limited information about this combination, but it may increase the risk of psychosis and excessive stimulation.
Notably, while there are no documented fatalities associated with 5-MeO-MiPT, it is essential to consider the potential for harm, especially when excessive doses are taken or when combined with substances such as MAOIs, RIMAs, stimulants, or agents that affect neurotransmitter reuptake or release, like serotonin and dopamine.
Legal status
Austria: The possession, production, and sale of 5-MeO-MiPT are prohibited under the Neue Psychoaktive Substanzen Gesetz (NPSG). However, individuals without the intent to distribute are unlikely to face prosecution.
Brazil: 5-MeO-MiPT is illegal to produce, sell, or possess and is listed on Portaria SVS/MS nº 344.
China: 5-MeO-MiPT is classified as a Category I psychotropic substance and is illegal to sell, buy, import, export, and manufacture.
Finland: 5-MeO-MiPT was banned in Finland in December 2014.
Germany: 5-MeO-MiPT is controlled under the New Psychoactive Substances Act (NpSG) as of July 18, 2019. Production and import to market, administering it to another person, marketing, and trading are punishable offenses. Possession is illegal but not subject to punishment. The legislator also considers the possibility that orders of 4-HO-MiPT may be punishable as incitement to market it.
Japan: 5-MeO-MiPT is designated as a controlled substance by the Pharmaceutical Affairs Law, making it illegal to possess or sell.
Latvia: 5-MeO-MiPT is classified as a Schedule I drug in Latvia.
Luxembourg: 5-MeO-MiPT is not a prohibited substance, making it legal.
New Zealand: 5-MeO-MiPT is considered an analog of DMT, classifying it as a Class C controlled drug.
Romania: 5-MeO-MiPT and its derivatives are illegal in Romania, effective January 2011.
Switzerland: 5-MeO-MiPT is categorized as a controlled substance and specifically named under Verzeichnis E.
United Kingdom: 5-MeO-MiPT is classified as a Class A drug due to its status as an ether of 5-HO-MiPT, itself a Class A drug under the tryptamine catch-all clause.
United States: 5-MeO-MiPT is not scheduled federally but could be considered an analog of 5-MeO-DiPT, a Schedule I drug under the Controlled Substances Act. Therefore, its sale for human consumption or illicit non-medical or industrial purposes may be prosecuted as a crime under the Federal Analogue Act.
- Florida: 5-MeO-MiPT is a Schedule I drug in Florida.
- Louisiana: 5-MeO-MiPT is a Schedule I drug, effective June 2013.
- Minnesota: Minnesota has banned a series of drugs, including 5-MeO-MiPT.
FAQ
1. What is 5-MeO-MiPT?
5-MeO-MiPT, or “moxy,” is a synthetic indole alkaloid belonging to the tryptamine class of compounds. It is a psychedelic substance with effects on serotonin receptors in the brain.
2. What are the effects of 5-MeO-MiPT?
The effects of 5-MeO-MiPT can vary from person to person, but commonly reported experiences include stimulation, entactogenic qualities, visual enhancements, and, in some cases, hallucinations. It can also affect mood and sensory perception.
3. Is 5-MeO-MiPT legal?
The legal status of 5-MeO-MiPT varies by country and region. It is illegal in some countries, while it may be unregulated or considered a controlled substance in others. Always check local laws and regulations before obtaining or using it.
4. What are the potential risks of using 5-MeO-MiPT?
While the long-term effects of 5-MeO-MiPT are not well understood due to limited research, there are potential risks associated with its use. These include unpredictable reactions, nausea, body load, and the risk of adverse psychological reactions. Combining it with other substances can also be dangerous.
5. Is 5-MeO-MiPT addictive?
5-MeO-MiPT is generally not considered habit-forming, and users often report a reduced desire to use it with continued use.
6. What is the recommended dosage of 5-MeO-MiPT?
Dosage can vary depending on the individual and their tolerance, but typical doses range from 5 to 10 milligrams when taken orally. Smoking or vaporizing may require smaller amounts. However, starting with a lower dose and exercising caution is crucial.
7. How long do the effects of 5-MeO-MiPT last?
The effects of 5-MeO-MiPT typically last for 4 to 6 hours when taken orally. Smoking or vaporizing the substance may produce shorter-lasting results.
8. Can 5-MeO-MiPT be detected in drug tests?
Standard drug tests typically do not screen for 5-MeO-MiPT. However, specialized tests may be able to detect its presence.
9. How can I reduce the risks associated with using 5-MeO-MiPT?
It is strongly recommended to practice harm reduction when using 5-MeO-MiPT. Start with a low dose, be aware of your surroundings, avoid combining it with other substances, and have a trusted friend or sitter present. Additionally, please educate yourself about the laws regarding its possession and use in your area.
References
- Repke, D. B.; Grotjahn, D. B.; Shulgin, A. T. (1985). “Psychotomimetic N-methyl-N-isopropyltryptamines. Effects of variation of aromatic oxygen substituents”. Journal of Medicinal Chemistry. 28 (7): 892–896. doi:10.1021/jm00145a007. eISSN 1520-4804. ISSN 0022-2623. OCLC 39480771. PMID 4009612.
- Shulgin, Alexander; Shulgin, Ann (1997). “#40. 5-MeO-MiPT”. TiHKAL: The Continuation. United States: Transform Press. ISBN 0-9630096-9-9. OCLC 38503252.
- Nagai, F.; Nonaka, R.; Satoh, K.; Kamimura, H. (2007). “The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain.” European Journal of Pharmacology. 559 (2–3): 132–137. doi:10.1016/j.ejphar.2006.11.075. eISSN 1879-0712. ISSN 0014-2999. OCLC 01568459. PMID 17223101.
- Ray, T. S. (March 4, 2010). “Correction: Psychedelics and the Human Receptorome.” PLoS ONE. 5 (3). doi:10.1371/annotation/e580a864-cf13-40c2-9bd9-b9687a6f0fe4. ISSN 1932-6203.
- Altuncı YA, Aydoğdu M, Açıkgöz E, Güven Ü, Düzağaç F, Atasoy A, Dağlıoğlu N, Annette Akgür S. New Psychoactive Substance 5-MeO-MiPT In vivo Acute Toxicity and Hystotoxicological Study. Balkan Med J. 2021 Jan;38(1):34-42. doi:10.4274/balkanmedj.galenos.2020.2019.11.68 PMID: 32936075; PMCID: PMC8909217.
- Gillman, P. K. (2005). “Monoamine oxidase inhibitors, opioid analgesics, and serotonin toxicity.” British Journal of Anaesthesia. 95 (4): 434–441. doi:10.1093/bja/aei210. eISSN 1471-6771. ISSN 0007-0912. OCLC 01537271.
- Gesamte Rechtsvorschrift für Neue-Psychoaktive-Substanzen-Gesetz (in German). Bundeskriminalamt Österreich. Retrieved April 13, 2023.
- “RESOLUÇÃO DA DIRETORIA COLEGIADA – RDC N° 130, DE 2 DE DEZEMBRO DE 2016” (in Portuguese). Agência Nacional de Vigilância Sanitária (ANVISA) [Brazilian Health Regulatory Agency (ANVISA)]. December 5, 2016.
- “Erowid China Psychoactive Law Update: September 2015” (PDF). Erowid. They were retrieved on September 29, 2020.
- “1130/2014: Valtioneuvoston asetus: kuluttajamarkkinoilta kielletyistä psykoaktiivisista aineista” (PDF). Suomen Säädöskokoelma (in Finnish). December 19, 2014.
- “Anlage NpSG” (in German). Bundesamt für Justiz [Federal Office of Justice]. Retrieved December 10, 2019.
- “Verordnung zur Änderung der Anlage des Neue-psychoaktive-Stoffe-Gesetzes und von Anlagen des Betäubungsmittelgesetzes” (PDF). Bundesgesetzblatt Jahrgang 2019 Teil I Nr. 27 (in German). Bundesanzeiger Verlag. July 17, 2019. pp. 1083–1094. ISSN 0341-1095.
- “§ 4 NpSG” (in German). Bundesamt für Justiz [Federal Office of Justice]. They were retrieved on December 10, 2019.
- “§ 3 NpSG” (in German). Bundesamt für Justiz [Federal Office of Justice]. They were retrieved on December 10, 2019.
- “Gesetzentwurf der Bundesregierung: Entwurf eines Gesetzes zur Bekämpfung der Verbreitung neuer psychoaktiver Stoffe” (PDF) (in German). Deutscher Bundestag. May 30, 2016. p. 20. Drucksache 18/8579.
- “Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām
