The availability of 5-MeO-DMT through various online sellers and vendors has raised concerns within the scientific and psychedelic communities. This potent designer drug, classified as a research chemical, has garnered attention for its powerful psychoactive effects, but its online sale and purchase present several issues that demand careful consideration.
First and foremost, the online sale of 5-MeO-DMT blurs the lines between legitimate research and recreational use. While research chemicals have value in scientific studies, their accessibility to the general public can lead to misuse and potential harm. Sellers must exercise caution in marketing and distributing such substances, as they can easily be abused by individuals seeking mind-altering experiences rather than conducting legitimate research.
Another concern is the need for more regulation and quality control in the production and distribution of 5-MeO-DMT. Some sellers may not adhere to strict manufacturing standards, potentially leading to variations in purity and potency. This poses significant risks to consumers who may inadvertently ingest substances of unknown origin and composition.
Furthermore, the online sale of 5-MeO-DMT often bypasses traditional medical and ethical safeguards. Users may not receive proper guidance or support, increasing the likelihood of adverse experiences and psychological distress. This unregulated market can put individuals at risk, especially those unprepared for the compound’s intense and profound effects.
Contents
- 1 Summary
- 2 Chemistry
- 3 Effects
- 4 Uses
- 5 Pharmacology
- 6 Clinical Development
- 7 Sources
- 8 Legal status
- 9 FAQ
- 9.1 1. What is 5-MeO-DMT?
- 9.2 2. How is 5-MeO-DMT different from DMT?
- 9.3 3. Is 5-MeO-DMT legal?
- 9.4 4. What are the effects of 5-MeO-DMT?
- 9.5 5. How is 5-MeO-DMT typically used?
- 9.6 6. What are the potential risks and side effects of 5-MeO-DMT?
- 9.7 7. Is 5-MeO-DMT addictive?
- 9.8 8. Can 5-MeO-DMT be used for therapeutic purposes?
- 9.9 9. How should I prepare if I’m considering using 5-MeO-DMT?
- 9.10 10. What should I do if I or someone I know experiences a problematic trip on 5-MeO-DMT?
- 10 References
Summary
5-MeO-DMT, scientifically known as 5-methoxy-N,N-dimethyltryptamine or O-methyl-bufotenin, belongs to the tryptamine class of psychedelics. It is naturally occurring in a diverse range of plant species and is also produced by the glands of at least one species of toad, specifically the Colorado River toad. Like its close chemical relatives, DMT and bufotenin (5-HO-DMT), 5-MeO-DMT has traditionally been employed as an entheogen in various cultural practices in South America. Some common slang terms for this substance include Five-methoxy, the power, bufo, and toad venom.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1019-45-0 |
|---|---|
| PubChem CID | 1832 |
| IUPHAR/BPS | 145 |
| ChemSpider | 1766 |
| UNII | X0MKX3GWU9 |
| KEGG | C08309 |
| ChEBI | CHEBI:2086 |
| ChEMBL | ChEMBL7257 |
| CompTox Dashboard (EPA) | DTXSID70144324 |
| ECHA InfoCard | 100.012.558 |
| Chemical and physical data | |
| Formula | C13H18N2O |
| Molar mass | 218.300 g·mol−1 |
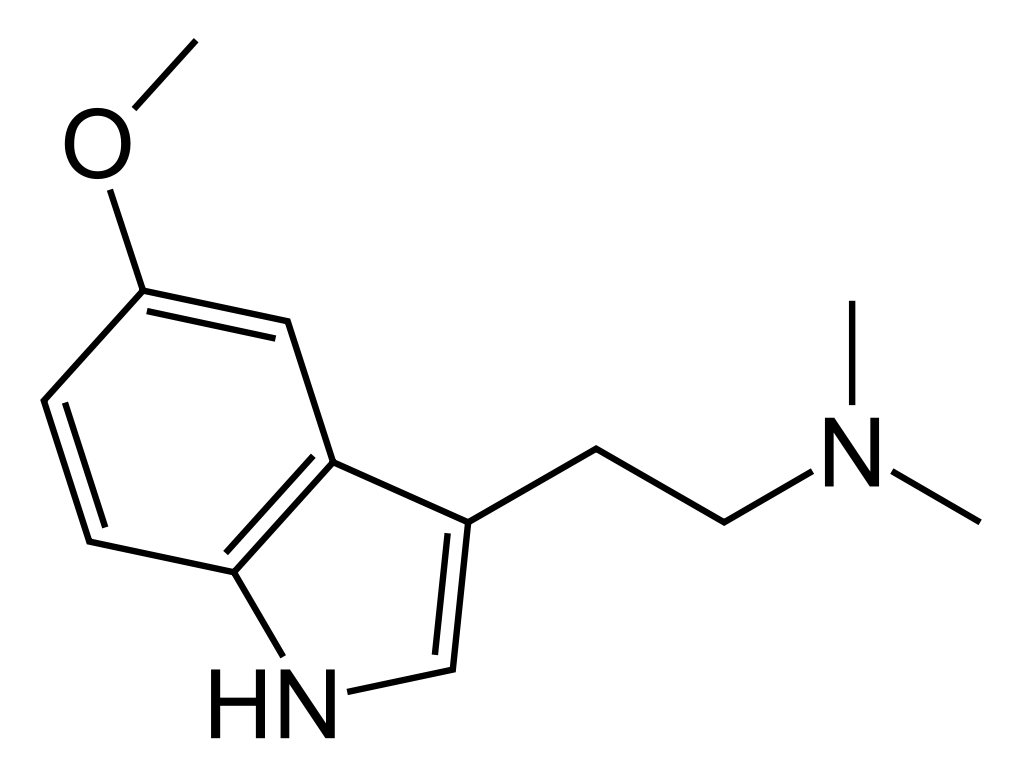
Chemistry
5-MeO-DMT was initially synthesized in 1936, and its psychoactive properties were discovered when it was isolated in 1959 as one of the active compounds found in Anadenanthera peregrina seeds, commonly used in the preparation of Yopo snuff. It was once believed to be a primary contributor to the psychoactive effects of the snuff. However, recent research has cast doubt on this assumption. This doubt arises from the limited or, in some cases, nonexistent amounts of 5-MeO-DMT present in the seeds. Instead, the psychoactivity of the sources appears to be primarily derived from bufotenin, the O-demethylated metabolite of 5-MeO-DMT. Notably, 5-MeO-DMT is metabolized mainly by the enzyme CYP2D6.
Effects
The duration of the experience with 5-MeO-DMT depends on the route of administration. When smoked, the total experience typically lasts around 10 minutes, whereas when insufflated (snorted), it can extend up to 2 hours. The effects are diverse and encompass a broad spectrum, including profound changes in perspective, the revelation of new insights, feelings of euphoria, immersive experiences, dissociation leading to non-responsiveness, heightened sensual and erotic sensations, as well as potential adverse effects such as dysphoria, fear, terror, and panic.
Uses
It exhibits anti-anxiety and anti-depressant properties.
Religious Usage: The Church of the Tree of Life, established in California in 1971 by John Mann (now defunct), officially recognized using 5-MeO-DMT as a sacrament. For approximately two decades, from around 1971 to the late 1980s, 5-MeO-DMT was discreetly accessible to its members. From 1970 to 1990, smoking 5-MeO-DMT on parsley was likely among the two most prevalent consumption methods in the United States.
Pharmacology
5-MeO-DMT, a methoxylated derivative of DMT, exhibits a distinct pharmacological profile compared to most common psychedelics. While many psychedelics are thought to primarily exert their psychological effects by activating serotonin 5-HT2A receptors, 5-MeO-DMT stands out for its exceptional affinity for 5-HT1A receptors, which is approximately 1000 times greater than its affinity for 5-HT2A receptors. This heightened affinity for 5-HT1A receptors aligns with its potent ability to inhibit the firing of dorsal raphe 5-HT neurons. Notably, experiments in rats have shown that the effects of 5-MeO-DMT were diminished when a 5-HT1A selective antagonist called WAY-100635 was administered, whereas a 5-HT2A selective antagonist called volinanserin had no discernible impact.
In addition to its interaction with serotonin receptors, 5-MeO-DMT’s mechanisms of action may also involve the inhibition of monoamine reuptake. A notable European study conducted in 2019 involving 42 volunteers revealed that a single inhalation of 5-MeO-DMT resulted in a sustained improvement in overall life satisfaction, along with reductions in anxiety, depression, and symptoms of post-traumatic stress disorder (PTSD). Furthermore, a study from 2018 demonstrated that a single dose of 5-MeO-DMT stimulated neurogenesis in mice, suggesting potential therapeutic implications.
Clinical Development
5-MeO-DMT is currently undergoing development and assessment for its potential therapeutic applications in patients with Treatment-Resistant Depression (TRD). GH Research, a biopharmaceutical company, has sponsored and successfully completed a phase 1 study involving healthy volunteers and a phase 1/2 study with TRD patients. Remarkably, during the phase 2 segment of the survey, 87.5% of TRD patients achieved remission by day 7. GH Research is presently planning a phase 2b trial with TRD patients. It has obtained approval to conduct studies involving patients with bipolar II disorder and a current depressive episode, as well as patients with postpartum depression.
Furthermore, Beckley Psytech, in collaboration with King’s College London, is conducting a phase 1 study to assess the safety and tolerability of intranasal 5-MeO-DMT in healthy subjects. Beckley Psytech’s CEO, Cosmo Feilding-Mellen, recognizes the potential benefits of 5-MeO-DMT’s short-acting nature compared to substances like psilocybin. Feilding-Mellen points out that the prolonged presence of therapists during an MDMA or psilocybin experience, which can span an entire working day, may be resource-intensive and costly. Considering the global shortage of psychotherapists, this could pose a potential bottleneck to patient access.
Sources
| Family | Animals |
|---|---|
| Bufonidae | Colorado River toad (Bufo alvarius) |
| Family | Fungi |
|---|---|
| Amanitaceae | Amanita citrina, Amanita porphyria |
Legal status
China: As of October 2015, 5-MeO-DMT was categorized as a controlled substance in China.
Australia: 5-MeO-DMT, a structural analog of N,N-dimethyltryptamine (DMT), falls under Schedule 9 as a prohibited substance by the Poisons Standard.
Sweden: The health ministry, Statens folkhälsoinstitut, classified 5-MeO-DMT, referred to as 5-metoxi-N,N-dimetyltryptamin (5-MeO-DMT) in their regulation SFS 2004:696, as a “health hazard” under the Act on the Prohibition of Certain Goods Dangerous to Health (Lagen om förbud mot vissa hälsofarliga varor) in October 2004, rendering it illegal to sell or possess.
Germany: 5-MeO-DMT has been listed as a controlled substance since 2001, specifically under Attachment I of the BtMG (Betäubungsmittelgesetz). This regulation is documented in BGBl. I 2001, 1180 – 1186.
Turkey: Turkey has controlled 5-MeO-DMT since December 2013.[34]
United States: 5-MeO-DMT was classified as a Schedule I controlled substance in January 2011 in the United States.
FAQ
1. What is 5-MeO-DMT?
- 5-MeO-DMT is a naturally occurring psychedelic compound found in various plants and the secretions of the Colorado River toad (Bufo alvarius). It is known for its powerful psychoactive effects.
2. How is 5-MeO-DMT different from DMT?
- While both are psychedelic, 5-MeO-DMT and DMT (N, N-Dimethyltryptamine) have distinct effects. 5-MeO-DMT is often described as producing a more intense, ego-dissolving experience that lacks the visual elaborate impact commonly associated with DMT.
3. Is 5-MeO-DMT legal?
- The legal status of 5-MeO-DMT varies by country and region. In many places, it is considered a controlled substance and is illegal to possess, sell, or use. Always check your local laws before considering its use.
4. What are the effects of 5-MeO-DMT?
- Effects of 5-MeO-DMT can include intense and rapid-onset psychedelic experiences, a sense of ego dissolution or unity with the universe, vivid sensory and emotional experiences, and profound shifts in perception. The effects can vary from person to person.
5. How is 5-MeO-DMT typically used?
- 5-MeO-DMT is most commonly smoked, vaporized, or insufflated (snorted). It can also be found in some traditional shamanic rituals, particularly in South America.
6. What are the potential risks and side effects of 5-MeO-DMT?
- Using 5-MeO-DMT carries risks, including intense psychological experiences that can be overwhelming or distressing. Physical hazards may include increased heart rate and blood pressure. Additionally, there is a risk of legal consequences due to its status as a controlled substance in many places.
7. Is 5-MeO-DMT addictive?
- There is limited research on the addictive potential of 5-MeO-DMT, but it is generally considered to have a low potential for physical dependence. However, psychological support is possible, especially in individuals prone to substance misuse.
8. Can 5-MeO-DMT be used for therapeutic purposes?
- Some research suggests that 5-MeO-DMT may have therapeutic potential for depression, anxiety, and addiction. However, more research is needed, and it should only be used in a therapeutic context under the guidance of trained professionals where legal.
9. How should I prepare if I’m considering using 5-MeO-DMT?
- If you are considering using 5-MeO-DMT, it’s essential to research the substance thoroughly, be aware of the legal status in your area, and consider potential risks. If you decide to proceed, ensure that you are in a safe and supportive environment and consider having a trusted trip sitter present.
10. What should I do if I or someone I know experiences a problematic trip on 5-MeO-DMT?
- If you or someone you know is having a challenging experience with 5-MeO-DMT, staying calm and reassuring is crucial. If the situation worsens, seek immediate medical attention if necessary. It’s essential to approach the substance cautiously and respect its potential effects.
References
- Anvisa (2023-07-24). “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Published in Diário Oficial da União on 2023-07-25. Archived from the original on 2023-08-27. Retrieved on 2023-08-27.
- Araújo AM, Carvalho F, Bastos M, Guedes de Pinho P, Carvalho M (August 2015). “The hallucinogenic world of tryptamines: an updated review.” Published in Archives of Toxicology, Volume 89 (8), Pages 1151–1173. doi:10.1007/s00204-015-1513-x. PMID 25877327. S2CID 4825078.
- “Ultimate Guide to 5-MeO-DMT – Experience, Benefits, & Side Effects”. They were published on 29 June 2020.
- Ott J (July–September 2001). “Pharmañopo-psychonauts: human intranasal, sublingual, intrarectal, pulmonary and oral pharmacology of bufotenine.” Published in the Journal of Psychoactive Drugs, Volume 33 (3), Pages 273–281. doi:10.1080/02791072.2001.10400574. PMID 11718320. S2CID 5877023.
- Shen HW, Jiang XL, Winter JC, Yu AM (October 2010). “Psychedelic 5-methoxy-N, N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions”. Published in Current Drug Metabolism, Volume 11 (8), Pages 659–666. doi:10.2174/138920010794233495. PMC 3028383. PMID 20942780.
- “5-MeO-DMT Effects by Erowid”. Retrieved on 2021-07-30 from Erowid.org.
- Davis AK, So S, Lancelotta R, Barsuglia JP, Griffiths RR (March 2019). “5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT) used in a naturalistic group setting is associated with unintended improvements in depression and anxiety”. Published in The American Journal of Drug and Alcohol Abuse, Volume 45 (2), Pages 161–169. doi:10.1080/00952990.2018.1545024. PMC 6430661. PMID 30822141.
- Davis AK, Barsuglia JP, Lancelotta R, Grant RM, Renn E (July 2018). “The epidemiology of 5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT) use: Benefits, consequences, patterns of use, subjective effects, and reasons for consumption”. Published in the Journal of Psychopharmacology, Volume 32 (7), Pages 779–792. doi:10.1177/0269881118769063. PMC 6248886. PMID 29708042.
- John Mann; Adam Gottlieb (2015) [First published 1970]. “back cover.” The Book of Sacraments: Ritual Use of Magical Plants. Ronin Publishing. ISBN 978-1-57951-210-1.
- “5-MeO-DMT Timeline” on Erowid.
- Ray TS (February 2010). “Psychedelics and the human receptorome”. Published in PLOS ONE, Volume 5 (2), Article e9019. doi:10.1371/journal.pone.0009019. PMC 2814854. PMID 20126400.
- Rogawski MA, Aghajanian GK (October 1981). “Serotonin autoreceptors on dorsal raphe neurons: structure-activity relationships of tryptamine analogs.” Published in J Neurosci, Volume 1 (10), Pages 1148–1154. doi:10.1523/JNEUROSCI.01-10-01148.1981. PMC 6564212. PMID 6793698.
- Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA (December 2006). “The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats”. Published in Psychopharmacology, Volume 189 (3), Pages 319–329. doi:10.1007/s00213-006-0566-1. PMID 17013638. S2CID 23396616.
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K (March 2007). “The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain.” Published in the European Journal of Pharmacology, Volume 559 (2–3), Pages 132–137. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
- Uthaug MV, Lancelotta R, van Oorsouw K, Kuypers KP, Mason N, Rak J, et al. (September 2019). “A single inhalation of vapor from dried toad secretion containing 5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT) in a naturalistic setting is related to sustained enhancement of satisfaction with life, mindfulness-related capacities, and a decrement of psychopathological symptoms”. Published in Psychopharmacology, Volume 236 (9), Pages 2653–2666. doi:10.1007/s00213-019-05236-w. PMC 6695371. PMID 30982127.
- Lima da Cruz RV, Moulin TC, Petiz LL, Leão RN (2018). “A Single Dose of 5-MeO-DMT Stimulates Cell Proliferation, Neuronal Survivability, Morphological and Functional Changes in Adult Mice Ventral Dentate Gyrus”. Published in Frontiers in Molecular Neuroscience, Volume 11, Article 312. doi:10.3389/fnmol.2018.00312. PMC 6131656. PMID 30233313.
- “Home | GH Research” by GH Research Limited.
- Reckweg J, Mason NL, van Leeuwen C, Toennes SW, Terwey TH, Ramaekers JG (2021). “A Phase 1, Dose-Ranging Study to Assess Safety and Psychoactive Effects of a Vaporized 5-Methoxy-N, N-Dimethyltryptamine Formulation (GH001) in Healthy Volunteers”. Published in Frontiers in Pharmacology, Volume 12, Article 760671. doi:10.3389/fphar.2021.760671. PMC 8667866. PMID 34912222.
- GH Research PLC (2021-12-06). “GH Research Announces Successful Outcome of the Phase 2 part of its Phase 1/2 Clinical Trial of GH001 in Treatment-Resistant Depression”. Retrieved on 2022-10-07 from GlobeNewswire News Room.
- GH Research PLC (2022-08-23). “GH Research Reports Second Quarter 2022 Financial Results and Provides Business Updates”. Retrieved on 2022-10-07 from GlobeNewswire News Room.
- Carpenter DE (2022-02-16). “More Companies Embrace 5-MeO-DMT to Create Therapies”. Retrieved on 2022-03-06 from Lucid News.
- Siebert A. “Could 5-MeO-DMT Allow For More Affordable Psychedelic-Assisted Therapy? Beckley Psytech Thinks So”. Retrieved on 2022-03-06 from Forbes.
- Sherwood AM, Claveau R, Lancelotta R, Kaylo KW, Lenoch K (December 2020). “Synthesis and Characterization of 5-MeO-DMT Succinate for Clinical Use”. Published in ACS Omega, Volume 5 (49), Pages 32067–32075. doi:10.1021/acsomega.0c05099. PMC 7745443. PMID 33344861.
- Carpenter DE (2021-02-02). “Psychedelic Toads Pushed To The Limit, Conservationists Urge Synthetic 5-MeO-DMT Option”. Retrieved on 2021-02-04 from Forbes.
- Uthaug MV, Lancelotta R, Szabo A, Davis AK, Riba J, Ramaekers JG (March 2020). “Prospective examination of synthetic 5-methoxy-N, N-dimethyltryptamine inhalation: effects on salivary IL-6, cortisol levels, affect, and non-judgment”. Published in Psychopharmacology, Volume 237 (3), Pages 773–785. doi:10.1007/s00213-019-05414-w. PMC 7036074. PMID 31822925.
- “Tryptamines: fungi” on bluezoo.org.
- “Erowid Psychoactive Vaults: Tryptamine FAQ” on www.erowid.org.
- “Some simple tryptamines” (PDF) on troutsnotes.com. They were retrieved on 2020-07-04.
- Khan JI, Kennedy TJ, Christian JR DR (2011). Basic Principles of Forensic Chemistry. Published by Springer Science & Business Media, Page 195. ISBN 978-1-934115-06-0.
- Carpenter DE. “5-MeO-DMT: The 20-Minute Psychoactive Toad Experience That’s Transforming Lives” on Forbes.
- “关于印发《非药用类麻醉药品和精神药品列管办法》的通知” (in Chinese) by China Food and Drug Administration. Published on 27 September 2015. Archived from the original on 1 October 2015 and retrieved on 1 October 2015.
- “Poisons Standard July 2016” in the Federal Register of Legislation.
- “Förordning om ändring i förordningen (1999:58) om förbud mot vissa hälsofarliga varor” (PDF) (in Swedish). Published on 7 September 2004.
- “Turkish Law” (PDF) in Resmi Gazete. Published on 16 December 2013.
- Drug Enforcement Administration (DEA), Department of Justice (December 2010). “Schedules of controlled substances: placement of 5-methoxy-N, N-dimethyltryptamine into Schedule I of the Controlled Substances Act. Final rule” (PDF) in Federal Register, Volume 75 (243), Pages 79296–79300. PMID 21171485.