Beautiful Plants For Your Interior
Summary
25I-NBOH (also known as NBOH-2CI, Cimbi-27, 2-C-I-NBOH) belongs to the group of derivatives derived from the phenethylamine hallucinogen 2C-I, initially discovered in 2006 by a team at Purdue University.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 919797-20-9 |
|---|---|
| PubChem CID | 10001761 |
| ChemSpider | 8177342 |
| UNII | W9G4BGW8K4 |
| CompTox Dashboard (EPA) | DTXSID80433993 |
| Chemical and physical data | |
| Formula | C17H20INO3 |
| Molar mass | 413.255 g·mol−1 |
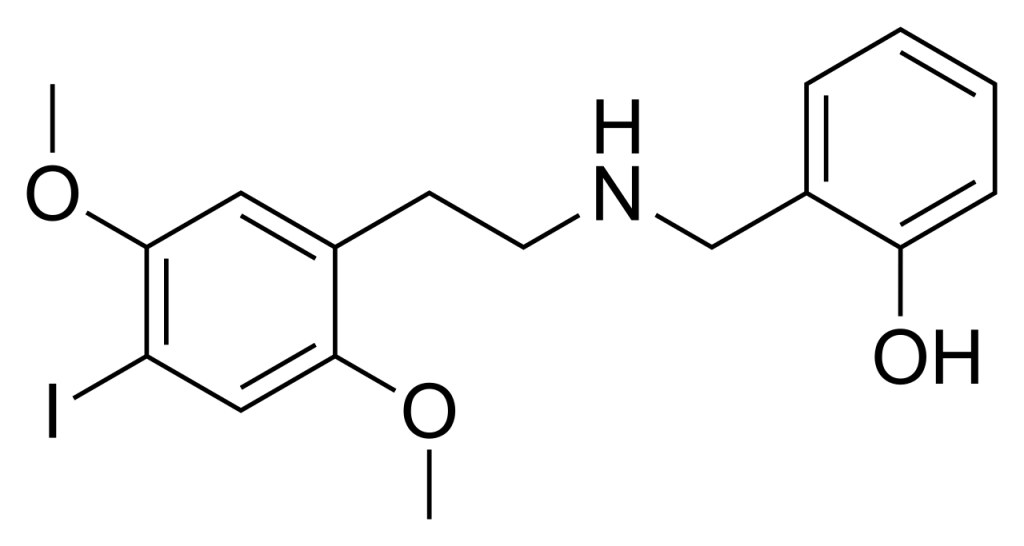
Pharmacology
25I-NBOH exhibits potent agonistic effects on the 5HT2A receptor, with a Ki of 0.061 nM at the human 5HT2A receptor, comparable to the well-known compound 25I-NBOMe, signifying its approximately twelvefold greater potency compared to 2C-I itself.
While in vitro tests demonstrate its agonistic behaviour, there have been no reported animal studies to validate these findings. Notably, the N-benzyl derivatives of 2C-I displayed significantly enhanced binding to 5HT2A receptor fragments relative to 2C-I, whereas the N-benzyl derivatives of DOI exhibited reduced activity compared to DOI.
Distinguished as one of the most selective agonist ligands for the 5-HT2A receptor, 25I-NBOH boasts an EC50 value of 0.074 nM, demonstrating more than 400 times selectivity over the 5-HT2C receptor.
Analytical chemistry
25I-NBOH is a labile molecule that readily breaks down into 2C-I when subjected to standard gas chromatography (GC) analysis methods. A dedicated approach for accurately identifying 25I-NBOH via GC/MS has been documented, enabling law enforcement agencies globally to distinguish this compound effectively.
Legality
Sweden
The Riksdag incorporated 25I-NBOH into the Narcotic Drugs Punishments Act [sv] under the Swedish schedule I (“substances, plant materials, and fungi which typically lack medical use”) effective from August 18, 2015, as published by the Medical Products Agency (MPA) in regulation HSLF-FS 2015:12, specified as “25I-NBOH” and “2-([2-(4-jodo-2,5-dimetoxifenyl)etylamino]metyl)fenol”.
United Kingdom
This substance falls under the Class A drug category in the United Kingdom due to including the N-benzyl phenethylamine catch-all clause in the Misuse of Drugs Act 1971.
FAQ
- What is 25I-NBOH? 25I-NBOH is a derivative of the phenethylamine-derived hallucinogen 2C-I that acts as a potent agonist of the 5HT2A receptor, known for its psychedelic properties.
- Is 25I-NBOH legal? The legal status of 25I-NBOH varies across different countries. It is important to check local regulations and laws regarding its use, possession, and distribution.
- What are the effects of 25I-NBOH? The effects of 25I-NBOH include psychedelic experiences, alterations in sensory perception, and changes in mood and thought patterns. Users may experience visual and auditory hallucinations and an altered sense of time.
- Are there any health risks associated with 25I-NBOH use? The use of 25I-NBOH may pose certain health risks, including potential adverse reactions, psychological distress, and, in some cases, severe toxic effects. It is essential to be aware of these risks before using the substance.
- How should 25I-NBOH be handled and stored? Proper handling and storage of 25I-NBOH are crucial to ensure its stability and prevent degradation. It should be stored in a secure and controlled environment away from direct light, moisture, and extreme temperatures.
- Can 25I-NBOH be detected in drug tests? Standard drug tests may be able to detect the presence of 25I-NBOH in biological samples. It is advisable to be aware of the substance’s detection window and consult with relevant authorities or professionals for accurate information.
- What precautions should be taken before using 25I-NBOH? It is essential to approach the use of 25I-NBOH with caution and awareness. Understanding the appropriate dosage, potential interactions with other substances or medications, and the importance of harm reduction practices is crucial.
- What should I do in case of an adverse reaction to 25I-NBOH? If you or someone you know experiences an adverse reaction to 25I-NBOH, seek immediate medical attention. Contact emergency services or a healthcare professional for appropriate guidance and assistance.
- Is there ongoing research on the effects of 25I-NBOH? Continued research is being conducted to further understand the effects, mechanisms of action, and potential therapeutic applications of 25I-NBOH. Stay updated with reliable scientific sources for the latest findings and developments in this field.
- Where can I find reliable information about 25I-NBOH? Seek information from reputable scientific publications, academic research, and government resources to access accurate and reliable information about 25I-NBOH. Be cautious of misinformation and unverified sources.
References
- Anvisa (2023-07-24). “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). This document was published in the Diário Oficial da União on 2023-07-25 and can be retrieved as it was archived from the original on 2023-08-27, with access to the archived version available until 2023-08-27.
- Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, et al. (April 2011). “Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers”. Published in the European Journal of Nuclear Medicine and Molecular Imaging (Vol. 38, No. 4), this research delves into the synthesis and evaluation of 11C-phenethylamines as PET tracers with 5-HT (2A) agonist properties. The study, indexed with PMID 21174090 and S2CID 12467684, provides insights into the use of these compounds.
- Silva ME, Heim R, Strasser A, Elz S, Dove S (January 2011). “Theoretical studies on the interaction of partial agonists with the 5-HT2A receptor”. This theoretical research, published in the Journal of Computer-Aided Molecular Design (Vol. 25, No. 1), examines the interaction of partial agonists with the 5-HT2A receptor, offering valuable insights. It was indexed with PMID 21088982 and S2CID 3103050.
- Braden MR, Parrish JC, Naylor JC, Nichols DE (December 2006). “Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists”. This study, published in Molecular Pharmacology (Vol. 70, No. 6), explores the molecular interactions of serotonin 5-HT2A receptor residues with superpotent N-benzyl phenethylamine agonists. The research is indexed with PMID 17000863 and S2CID 15840304.
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (March 2014). “Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists”. Published in ACS Chemical Neuroscience (Vol. 5, No. 3), this research discusses the synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists. The study can be referred to with PMID 24397362.
- Arantes LC, Júnior EF, de Souza LF, Cardoso AC, Alcântara TL, Lião LM, et al. (2017). “2A receptor agonist identified in blotter paper seizures in Brazil”. This work, featured in Forensic Toxicology (Vol. 35, No. 2), reports the identification of a 2A receptor agonist in blotter paper seizures in Brazil. It was published in 2017 and indexed with PMID 28706567.
- Neto JC, Andrade AF, Lordeiro RA, Machado Y, Elie M, Júnior EF, Arantes LC (2017). “Preventing misidentification of 25I-NBOH as 2C-I on routine GC–MS analyses.” This study, published in Forensic Toxicology (Vol. 35, No. 2), focuses on preventing the misidentification of 25I-NBOH as 2C-I in routine GC–MS analyses. It was indexed with S2CID 32432586.
- “Gemensamma författningssamlingen avseende hälso- och sjukvård, socialtjänst, läkemedel, folkhälsa m.m.” (PDF). Lakemedelsverket. This document, provided by Lakemedelsverket in Swedish, offers information regarding health care, social services, pharmaceuticals, and public health. It is available as a PDF.
- “The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014”. This legislative document, available online, pertains to the Misuse of Drugs Act 1971 and specifically addresses