Beautiful Plants For Your Interior
Summary
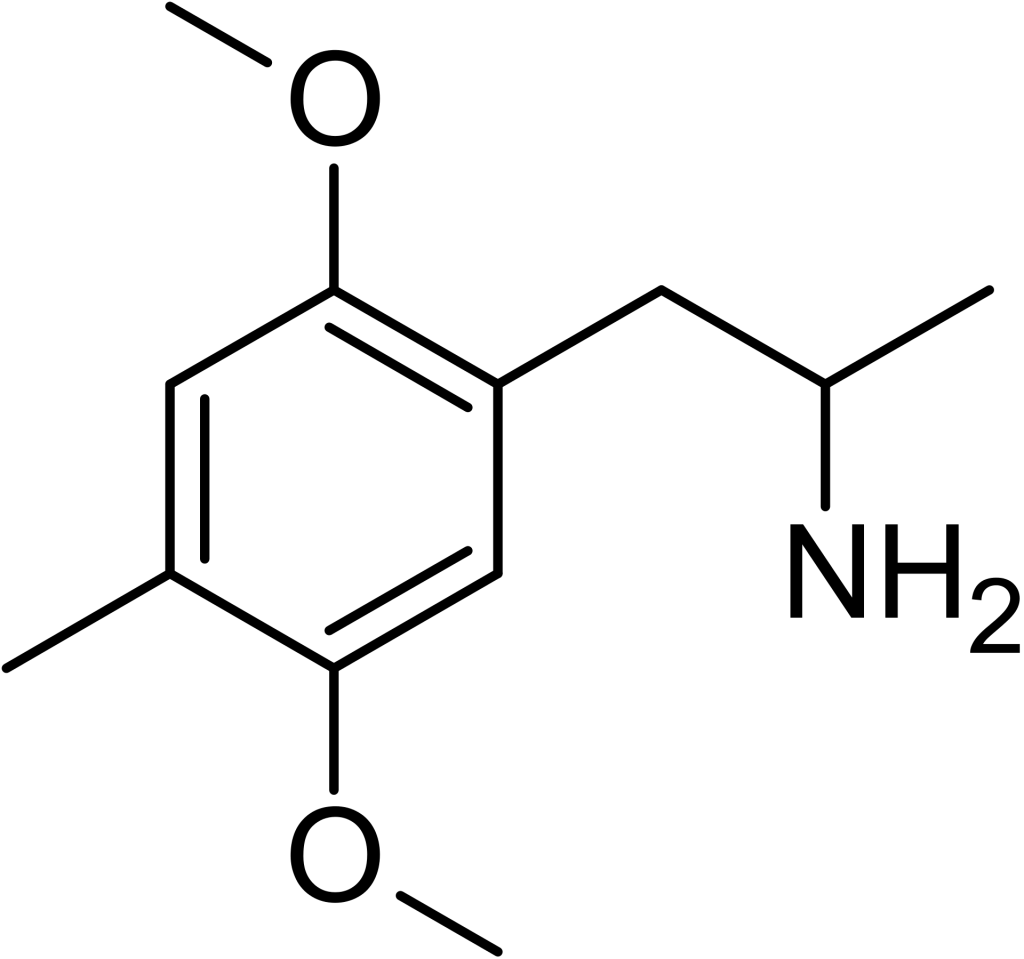
2,5-Dimethoxy-4-methylamphetamine (DOM), also recognized by the acronym STP (representing “Serenity, Tranquility, and Peace”), belongs to the class of psychedelics and substituted amphetamines. Renowned chemist Alexander Shulgin was the first to synthesize this compound, later documenting its properties in his famous book, PiHKAL: A Chemical Love Story. DOM is classified as a Schedule I substance in the United States and is subject to similar regulatory controls in other regions globally. Furthermore, it holds the status of a Schedule I drug internationally under the Convention on Psychotropic Substances. This compound is commonly ingested orally.
| Identifiers | |
|---|---|
| CAS Number | 15588-95-1 43061-13-8 (R) 43061-14-9 (S) |
| 3D model (JSmol) | Interactive imageR–isomer: Interactive image |
| ChEMBL | ChEMBL317634 |
| ChemSpider | 9910656 |
| PubChemCID | 11735949 |
| UNII | UKI9MLD5OI LX3MC6OB9X (R) 0FRQ2JVN98 (S) |
| CompTox Dashboard(EPA) | DTXSID50860611 |
History
The genesis of STP traces back to 1963 when Alexander Shulgin synthesized and examined its effects, focusing on the impact of 4-position substitutions on psychedelic amphetamines.
During the mid-1967 period, STP tablets containing 20 mg (later reduced to 10 mg) were widely circulated in San Francisco’s Haight-Ashbury District, courtesy of underground chemists Owsley Stanley and Tim Scully. However, the street presence of STP was short-lived and fraught with detrimental consequences. The tablets contained an excessively high dosage, coupled with DOM’s slow onset of action, leading some users to mistakenly dose, assuming effects would be immediate like those of LSD. Consequently, this miscalculation resulted in panicked users, with several requiring emergency medical treatment. Complicating matters further, the medical community was unaware that the tablets labeled as STP were, in reality, DOM.
Effects
The impacts of this substance encompass significant alterations in perception, leading to effects such as blurred vision, visual distortions, the appearance of multiple images, object vibrations, and enhanced detailing. Other products involve the distortion of shapes, a slowed perception of time, heightened sexual drive and pleasure, and increased sensory contrasts. Additionally, users may encounter mystical experiences and a shift in consciousness. Physiological responses can include pupillary dilation and an elevation in systolic blood pressure.
Pharmacology
DOM exhibits selectivity as a partial agonist of the 5-HT2A, 5-HT2B, and 5-HT2C receptors. Its psychedelic effects primarily stem from its agonistic interactions with the 5-HT2A receptor. This specificity makes DOM valuable in scientific investigations focusing on the 5-HT2 receptor subfamily. The chiral nature of DOM highlights R-(−)-DOM as the more potent enantiomer, effectively serving as a robust agonist of the serotonin 5-HT receptor family, particularly the 5-HT2 subtype.
Within the domain of DOM analogs and derivatives, the 2,6-dimethoxy positional isomer, known as Ψ-DOM, demonstrates activity, as does the alpha-ethyl homolog Ariadne, as mentioned in PiHKAL. Efforts to identify DOM’s binding mode at the 5-HT2A receptor have led to synthesizing and examining analogs with modifications at the 2,5- positions of the aromatic ring. Variants, including the 2- and 5- O-desmethyl derivatives (2-DM-DOM and 5-DM-DOM) and the 2- and 5- ethyl analogs (2-Et-DOM and 5-Et-DOM), have been studied. However, their potency was notably lower than that of the corresponding methoxy compound, emphasizing the critical role of oxygen lone pairs in 5-HT2A binding.
Toxicity
The toxicity profile of DOM remains largely unexplored. Information on its potential toxic effects is limited. As per Alexander Shulgin, the typical duration of DOM’s effects spans 14 to 20 hours, although other clinical trials suggest a shorter period of 7 to 8 hours.
Legal status
Canada
Designated as a Schedule 1 substance due to its structural similarity to amphetamine.
United States
DOM is classified as a Schedule I substance in the United States, rendering its manufacture, purchase, possession, or distribution illegal without a DEA license.
Australia
Under the Australia Poisons standard, DOM is categorized as schedule 9. This classification implies that it is a substance with potential for misuse or abuse, and its production, possession, sale, or use is generally prohibited by law. However, exceptions can be made for medical or scientific research or analytical, teaching, or training purposes with approval from Commonwealth and State or Territory Health Authorities.
United Kingdom
Regarded as a Class A drug in the United Kingdom, it falls under the jurisdiction of the Misuse of Drugs Act 1971.
FAQ
- What is 2,5-Dimethoxy-4-Methylamphetamine (DOM)?
- 2,5-Dimethoxy-4-Methylamphetamine, commonly called DOM, is a psychoactive compound classified as a substituted amphetamine. It is known for its psychedelic effects and is often used in scientific research.
- Who first synthesized DOM?
- DOM was initially synthesized and extensively studied by Alexander Shulgin, a renowned chemist known for his work in psychoactive compounds. His findings on DOM are documented in his book “PiHKAL: A Chemical Love Story.”
- What are the effects of DOM?
- The effects of DOM can include substantial changes in perception, such as visual distortions, blurred vision, multiple images, vibrational experiences, and enhanced details. Users may also experience a slowed sense of time, heightened sexual drive and pleasure, increased contrasts, mystical experiences, and changes in consciousness. Physiological effects can involve pupillary dilation and an increase in systolic blood pressure.
- Is DOM widely used recreationally?
- DOM has limited recreational use due to its potent and long-lasting effects. It is not as commonly used as other psychedelics like LSD. Also, confusion and potential health risks have arisen when DOM is sold under false names or misrepresented as other substances.
- How is DOM classified in different countries?
- Canada: DOM is listed as a Schedule 1 substance because it shares structural similarities with amphetamines.
- United States: DOM is classified as a Schedule I substance, making its manufacture, purchase, possession, or distribution illegal without a DEA license.
- Australia: It is categorized as Schedule 9 under the Australia Poisons standard. This implies that its production, possession, sale, or use is generally prohibited by law, except for specific research or educational purposes with the appropriate approvals.
- United Kingdom: DOM is considered a Class A drug in the United Kingdom, subject to the Misuse of Drugs Act 1971.
- What is the typical duration of DOM’s effects?
- The duration of DOM’s effects can vary. According to Alexander Shulgin, they typically last between 14 to 20 hours. However, in some cases, clinical trials have reported a shorter duration of 7 to 8 hours.
- Is DOM being studied for medical or therapeutic purposes?
- While DOM is primarily known for its recreational and research use, there is limited research exploring its potential therapeutic applications. Its prolonged duration of action and psychedelic properties could hold some potential for mental health, but further research is needed.
- What are some analogs and derivatives of DOM?
- Various analogs and derivatives of DOM have been synthesized and studied, including the 2,6-dimethoxy positional isomer known as Ψ-DOM and the alpha-ethyl homolog Ariadne. These compounds are explored as part of research to better understand DOM’s binding mode at the 5-HT2A receptor.
References
- Anvisa’s Regulatory Control: In Brazil, Anvisa (Agência Nacional de Vigilância Sanitária) controls substances under special regulation through resolutions like RDC Nº 804, which lists narcotic, psychotropic, precursor, and other substances. This is done to ensure the safe and responsible use of such substances in the country.
- International Psychotropic Substance Control: The International Narcotics Control Board issues a “Green List” of psychotropic substances under international control. This list plays a vital role in international efforts to regulate and monitor the use of such substances, contributing to global drug control policies.
- Alexander Shulgin’s Contributions: Alexander Shulgin, a prominent figure in the study of psychoactive compounds, documented his research and findings in his book “Pihkal: A Chemical Love Story.” His work has been instrumental in advancing our understanding of various substances, including 2,5-Dimethoxy-4-Methylamphetamine (DOM).
- Discovery of STP (DOM Variant): In 1967, STP (2,5-Dimethoxy-4-Methylamphetamine) gained recognition as a new hallucinogenic drug, with its effects and properties documented in the scientific journal “Science.” This marked an important moment in the study of psychoactive compounds.
- Serotonin Receptors and Partial Agonists: Research has shown that compounds like DOM are partial agonists at serotonin receptors, particularly those linked to phosphoinositide hydrolysis. This knowledge is crucial in understanding how these substances interact with the brain’s neurotransmitter systems.
- Metabolites and Behavioral Effects: The study of DOM metabolites has contributed to a better understanding of the behavioral effects of this substance and its derivatives. This research is essential in assessing the impact and potential risks associated with their use.
- Biophysical Basis of Hallucinogen Action: Ongoing research aims to uncover the biophysical basis of hallucinogenic actions, shedding light on the mechanisms by which substances like DOM affect the brain and induce hallucinatory experiences.
- Australia’s Poison Standard: Australia’s Poison Standard provides guidelines for the classification and regulation of various substances. It specifies how certain substances, including psychoactive compounds, should be handled, used, and controlled within the country.