Beautiful Plants For Your Interior
Summary
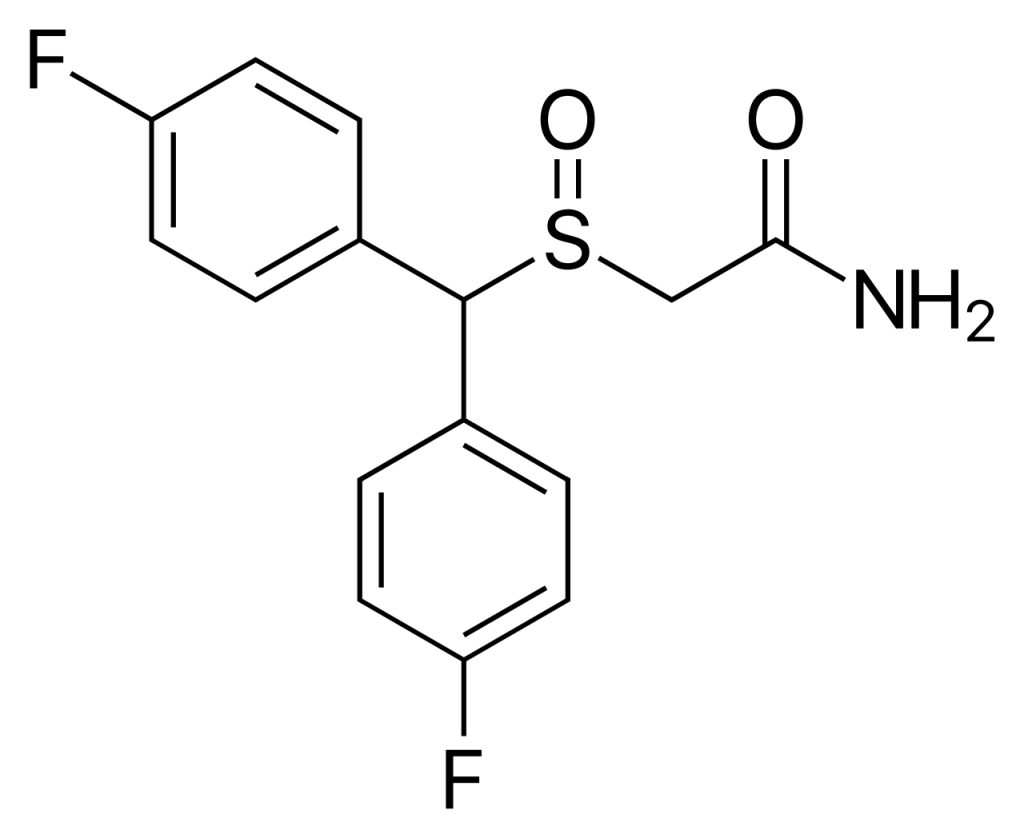
CRL-40,940, also referred to as flmodafinil, bisfluoromodafinil, and lauflumide, represents the bisfluoro derivative of modafinil. This compound falls under the category of eugeroics and functions as a mild dopamine reuptake inhibitor. Its developers assert that it exhibits superior efficacy to modafinil and adrafinil while presenting a reduced risk of side effects. CRL-40,940 was patented in 2013, and pre-clinical trials have been in progress since December 2015.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 90280-13-0 |
|---|---|
| PubChem CID | 13271852 |
| ChemSpider | 26386803 |
| UNII | 174R2IG4T0 |
| CompTox Dashboard (EPA) | DTXSID00533646 |
| Chemical and physical data | |
| Formula | C15H13F2NO2S |
| Molar mass | 309.33 g·mol−1 |
FAQ
- What is CRL-40,940?
- CRL-40,940, also known as flmodafinil, bisfluoromodafinil, and lauflumide, is a chemical compound and a derivative of modafinil. It is categorized as a eugeroic and functions as a weak dopamine reuptake inhibitor.
- How does CRL-40,940 differ from modafinil and adrafinil?
- CRL-40,940 is claimed by its inventors to be more effective than both modafinil and adrafinil while also having fewer associated side effects.
- What is a eugeroic?
- A eugeroic is a class of drugs that promotes wakefulness and alertness. They are often used to treat narcolepsy and excessive daytime sleepiness.
- What are the potential applications of CRL-40,940?
- CRL-40,940 is being explored in pre-clinical trials, and its potential applications may include treating sleep-related disorders and enhancing wakefulness and cognitive function.
- Is CRL-40,940 available for medical or recreational use?
- As of my knowledge, the cutoff date in September 2021, CRL-40,940, had not received regulatory approval for medical use. It is important to stay informed about its legal status in your region, as it may have changed since then.
- Are there any reported side effects of CRL-40,940?
- While its inventors claim fewer side effects than modafinil, it’s essential to consider that the safety profile of CRL-40,940 is still under investigation in pre-clinical trials. Potential side effects may become clearer as more research is conducted.
- Where can I find more information about CRL-40,940?
- To access comprehensive information about CRL-40,940, consider referring to scientific literature, research studies, and trustworthy sources. Additionally, stay updated on any developments regarding its regulatory status and potential medical applications.
References
- CA 1199916: Lafon L, “Benzhydrylsulfinylacetamide derivatives,” published on January 28, 1986.
- Cao J, Prisinzano TE, Okunola OM, Kopajtic T, Shook M, Katz JL, Newman AH (October 2010). “Structure-Activity Relationships at the Monoamine Transporters for a Novel Series of Modafinil (2-[(diphenylmethyl)sulfinyl]acetamide) Analogues.” Published in ACS Medicinal Chemistry Letters, Volume 2, Issue 1, Pages 48–52. DOI: 10.1021/ml1002025. PMC: 3041981. PMID: 21344069.
- US 20130295196: Konofal E, “Lauflumide and the enantiomers thereof, method for preparing same and therapeutic uses thereof,” published on November 7, 2013.
- “Flmodafinil Dosage: How Much Should You Take and When?” Accessed from modaforum.org. Retrieved on September 26, 2023.
- Louis Lafon (December 18, 1984). “Patent US 4489095 A – Halogenobenzhydrylsulfinylacetohydroxamic acids.” Retrieved on July 25, 2015.
- “Pipeline.” NLS Pharmaceuticals.