Beautiful Plants For Your Interior
Summary
Parahexyl, also known as Synhexyl or n-hexyl-Δ3-THC, is a synthetic counterpart to THC (tetrahydrocannabinol). It was developed in 1941 as part of efforts to decipher the chemical structure of Δ9-THC, one of the primary active compounds found in cannabis.
Parahexyl closely resembles THC in terms of both its structure and its effects, with the only difference being a shift in the position of one double bond and the extension of the 3-pentyl chain by one additional CH2 group, resulting in n-hexyl. When administered, Parahexyl induces effects typical of other compounds that activate cannabinoid receptors in animals. It exhibits slightly better oral bioavailability than THC but is otherwise quite similar in its actions. It is presumed to function as a CB1 receptor agonist, akin to THC. However, it’s important to note that research has yet to be published on Parahexyl since the discovery of the CB1 receptor, so this mechanism of action remains unconfirmed.
During the mid-20th century, Parahexyl was occasionally used as an anxiolytic, with dosages typically ranging from 5 mg to 90 mg.
In 1982, Parahexyl was classified as illegal under the UN convention due to its structural resemblance and similar effects profile to THC. It was placed in Schedule 1, the most restrictive category, categorizing it as a compound with no recognized medical utility despite the now well-established medical applications of cannabinoids.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 117-51-1 |
|---|---|
| PubChem CID | 8334 |
| ChemSpider | 8031 |
| UNII | 450N174F9W |
| Chemical and physical data | |
| Formula | C22H32O2 |
| Molar mass | 328.496 g·mol−1 |
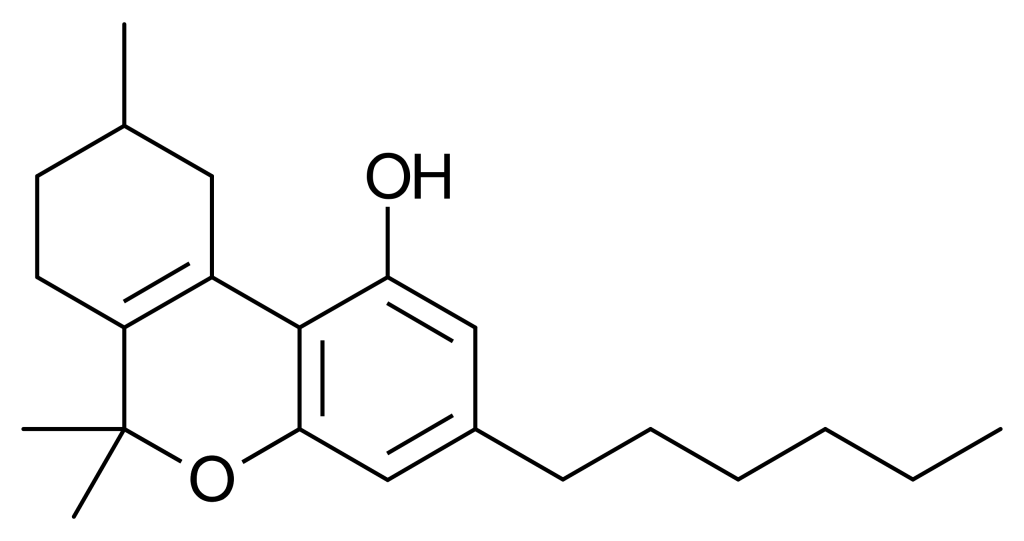
Isomerism
Several isomers of parahexyl have been subject to research and are recognized for their cannabinoid activity. Parahexyl itself, specifically the Δ6a(10a) isomer, has not been extensively explored in scientific investigations since its international ban in the early 1980s. However, both the Δ8 and Δ9 isomers have been identified as cannabinoid receptor agonists. Δ8-parahexyl is assigned the code number JWH-124, while Δ9-parahexyl has been extracted from Cannabis plant material and named tetrahydrocannabinol.
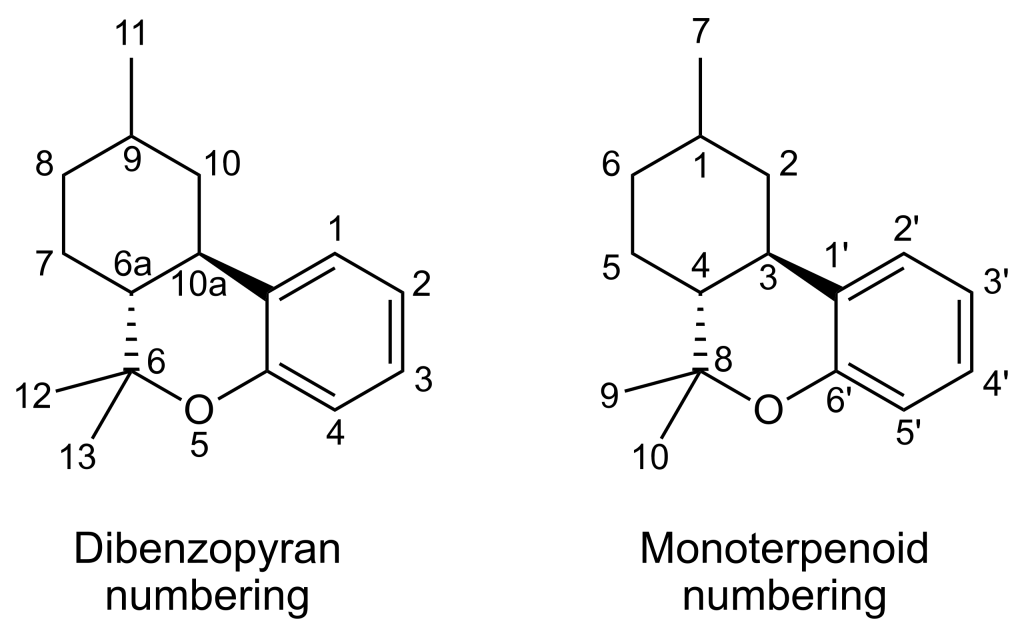
| 7 double bond isomers of parahexyl and their 30 stereoisomers | |||||||
|---|---|---|---|---|---|---|---|
| Dibenzopyran numbering | Monoterpenoid numbering | Number of stereoisomers | Natural occurrence | Convention on Psychotropic Substances Schedule | |||
| Short name | Chiral centers | Full name | Short name | Chiral centers | |||
| Δ6a(7)-parahexyl | 9 and 10a | 3-hexyl-8,9,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ4-parahexyl | 1 and 3 | 4 | No | unscheduled |
| Δ7-parahexyl | 6a, 9 and 10a | 3-hexyl-6a,9,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ5-parahexyl | 1, 3 and 4 | 8 | No | unscheduled |
| Δ8-parahexyl | 6a and 10a | 3-hexyl-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ6-parahexyl | 3 and 4 | 4 | No | unscheduled |
| Δ9,11-parahexyl | 6a and 10a | 3-hexyl-6a,7,8,9,10,10a-hexahydro-6,6-dimethyl-9-methylene-6H-dibenzo[b,d]pyran-1-ol | Δ1(7)-parahexyl | 3 and 4 | 4 | No | unscheduled |
| Δ9-parahexyl | 6a and 10a | 3-hexyl-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ1-parahexyl | 3 and 4 | 4 | No | unscheduled |
| Δ10-parahexyl | 6a and 9 | 3-hexyl-6a,7,8,9-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ2-parahexyl | 1 and 4 | 4 | No | unscheduled |
| Δ6a(10a)-parahexyl | 9 | 3-hexyl-7,8,9,10-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran-1-ol | Δ3-parahexyl | 1 | 2 | No | Schedule I |
FAQ
1. What is Parahexyl?
- Parahexyl is a synthetic compound developed as a counterpart to THC (tetrahydrocannabinol), one of the primary active components in cannabis. It was created for scientific research purposes.
2. How does Parahexyl relate to THC?
- Parahexyl closely resembles THC in both its chemical structure and its effects. The primary distinction is the position of a double bond and the extension of a carbon chain, resulting in n-hexyl.
3. What is the current status of Parahexyl in scientific research?
- Parahexyl (Δ6a(10a) isomer) has not been extensively studied since the early 1980s when it was banned internationally. However, other isomers, such as Δ8 and Δ9, are known to act as cannabinoid receptor agonists.
4. What are the known isomers of Parahexyl with cannabinoid activity?
- The Δ8 isomer is designated as JWH-124, and the Δ9 isomer has been isolated from Cannabis plant material and is known as tetrahydrocannabinol.
5. Why was Parahexyl banned internationally in the early 1980s?
- Parahexyl was classified as illegal under the UN convention due to its structural similarity and similar effects profile to THC. It was placed in Schedule 1, which categorizes it as a compound with no recognized medical utility.
6. Has Parahexyl ever been used for medical purposes?
- Parahexyl was occasionally employed as an anxiolytic in the mid-20th century, with dosages typically ranging from 5 mg to 90 mg.
7. Is Parahexyl used recreationally or commercially?
- Parahexyl is not used for recreational or commercial purposes. It was primarily developed for research and scientific investigations.
8. Can Parahexyl be legally obtained or prescribed?
- Parahexyl is generally illegal and is not available for legal prescription or use in many countries due to its classification as a Schedule 1 substance under international conventions.
9. Are there any known medical applications for Parahexyl or its isomers?
- While there are recognized medical applications for certain cannabinoids, such as those found in the Cannabis plant, Parahexyl itself has not been established for medical use. The focus in this regard is on cannabinoids like THC and CBD.
10. Is Parahexyl related to medical marijuana or CBD products?
- Parahexyl is a synthetic compound distinct from naturally occurring cannabinoids found in medical marijuana or CBD products. It was created for research purposes and does not have the exact natural origins.
References
- Anvisa, the Brazilian National Health Surveillance Agency, issued RDC Nº 804, a resolution detailing Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control. This document, published in Brazilian Portuguese, serves as a critical regulatory guideline. It can be found in the Diário Oficial da União, dated 2023-07-24, with archives available until 2023-08-27. To stay informed, refer to the original source.
- In 1941, a significant development occurred in the field of cannabinoids when researchers Adams, et al., explored Tetrahydrocannabinol Homologs with Marihuana Activity, as detailed in the Journal of the American Chemical Society, Volume 63, Issue 7. Their pioneering work marked a key milestone in cannabinoid research.
- Continuing this scientific exploration, Adams, Harfenist, and Loewe in 1949 delved into “New Analogs of Tetrahydrocannabinol,” as reported in the Journal of the American Chemical Society, Volume 71, Issue 5. Their findings contributed to our understanding of cannabinoid analogs.
- For expert insights into these compounds, don’t miss “Ask Dr. Shulgin Online,” a valuable resource for cannabinoid-related inquiries. Dr. Shulgin’s expertise is widely respected in the field.
- In 1974, Ono, Shimamine, Takahashi, and Inoue conducted research on hallucinogens, including the synthesis of parahexyl. Their work was published in “Eisei Shikenjo Hokoku,” the Bulletin of the National Institute of Hygienic Sciences.
- In 1980, Fairchild, Jenden, Mickey, and Yale conducted research on the EEG effects of hallucinogens and cannabinoids, using sleep-waking behavior as a baseline. Their findings were published in “Pharmacology, Biochemistry, and Behavior,” revealing crucial insights into the neurological effects of these substances.
- Supniewski’s 1950 publication “Farmakologia” provides valuable insights into the pharmacology of these compounds, offering foundational knowledge in the field.
- Martin, Jefferson, Winckler, Wiley, Huffman, Crocker, Saha, and Razdan’s research in 1999 on the manipulation of the tetrahydrocannabinol side chain paved the way for a nuanced understanding of agonists, partial agonists, and antagonists.
- Bow and Rimoldi’s work in 2016 contributed to understanding the structure-function relationships of classical cannabinoids and their modulation of CB1 and CB2 receptors.
- In December 2020, Linciano, Citti, Russo, Tolomeo, Laganà, Capriotti, Luongo, Iannotta, Belardo, Maione, Forni, Vandelli, Gigli, and Cannazza identified a new cannabidiol n-hexyl homolog, named cannabidihexol, in a medicinal cannabis variety. Their findings included its antinociceptive activity in mice and were reported in “Scientific Reports.”