Beautiful Plants For Your Interior
Summary
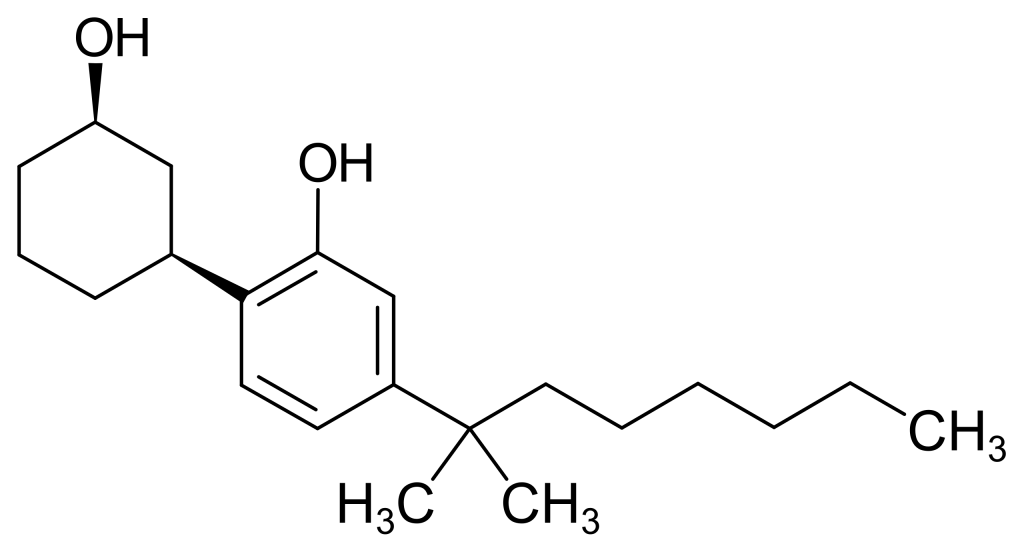
CP 47,497, also known as (C7)-CP 47,497, is a cannabinoid receptor agonist medication initially crafted by Pfizer during the 1980s. This compound, valued for its analgesic properties, is crucial in scientific investigations. Notably, CP 47,497 is a robust CB1 agonist with a Kd value of 2.1 nM.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | (1S,3R): 70434-82-1 |
|---|---|
| PubChem CID | (1S,3R): 125835(1R,3S): 15942731 |
| ChemSpider | (1S,3R): 10205286(1R,3S): 111910 |
| UNII | (1S,3R): FAPQAUKC04 |
| ChEMBL | (1S,3R): ChEMBL163701 |
| CompTox Dashboard (EPA) | (1S,3R): DTXSID401009968 |
| Chemical and physical data | |
| Formula | C21H34O2 |
| Molar mass | 318.501 g·mol−1 |
Legal status
Germany:
On January 22, 2009, CP 47,497, along with its dimethylhexyl, dimethyloctyl, and dimethylnonyl homologs, was included in the German controlled drug schedules (“Betäubungsmittelgesetz”).
France:
CP 47,497 and its C6, C8, and C9 homologs were prohibited in France on February 24, 2009.
Latvia:
Latvia banned CP 47,497 and its C6, C8, and C9 homologs on November 28, 2009.
Lithuania:
On June 5, 2009, Lithuania made CP 47,497 and its C6, C8, and C9 homologs illegal.
Sweden:
CP 47,497 and its C6, C7, C8, and C9 homologs were outlawed in Sweden on September 15, 2009.
Romania:
Romania banned CP 47,497 and its C6, C7, C8, and C9 homologs on February 15, 2010. (“Illegal Substances in Romania after 15.02.2010”. Archived from the original on 2010-03-27.)
United States:
As of March 1, 2011, CP 47,497 is classified as a Schedule 1 drug in the United States.
FAQ
- What is CP 47,497?
- CP 47,497 is a synthetic compound known as a cannabinoid receptor agonist. It was developed by Pfizer in the 1980s and has garnered attention for its analgesic properties.
- Why is CP 47,497 used in scientific research?
- CP 47,497 is utilized in scientific research due to its ability to interact with cannabinoid receptors, particularly CB1. This interaction provides insights into the endocannabinoid system and its potential applications.
- What is the legal status of CP 47,497 in different countries?
- The legal status of CP 47,497 varies by country. For example, it was added to the controlled drug schedules in Germany, France, Latvia, Lithuania, Sweden, and Romania at different times. In the United States, it was classified as a Schedule 1 drug as of March 1, 2011.
- Is CP 47,497 psychoactive?
- CP 47,497 is not typically described as psychoactive. Its primary use is for its analgesic effects and role in scientific research rather than producing a “high.”
- Are there any potential side effects associated with CP 47,497?
- Specific side effects related to CP 47,497 use may vary, and it’s essential to consult scientific literature or relevant authorities for detailed information.
- Can individuals access CP 47,497 for personal use?
- The availability and legality of CP 47,497 for personal use can differ by jurisdiction. It is crucial to be aware of local laws and regulations regarding its possession and use.
- What is the significance of CP 47,497 as a CB1 agonist?
- CP 47,497’s role as a CB1 agonist is significant for researchers studying the endocannabinoid system. It helps researchers understand how compounds interact with specific cannabinoid receptors and their potential therapeutic applications.
- Is CP 47,497 related to other cannabinoids, such as THC or CBD?
- CP 47,497 is a synthetic compound and not directly related to natural cannabinoids like THC or CBD. However, its interaction with cannabinoid receptors makes it part of the broader cannabinoid family in terms of its pharmacological effects.
- Where can I find more information about CP 47,497 for research or medical purposes?
- Researchers and medical professionals interested in CP 47,497 can refer to scientific journals, medical literature, or relevant authorities to access comprehensive information about its properties, applications, and legal status.
- Is CP 47,497 subject to ongoing research and development?
- The field of cannabinoid research is continually evolving, and compounds like CP 47,497 may continue to be studied for their potential therapeutic benefits and safety. Staying informed about the latest research is essential for those interested in this field.
References
- Weissman A, Milne GM, Melvin LS (November 1982). “Discovery of Cannabimimetic Effects from CP-47,497: A 3-Phenylcyclohexanol Derivative.” Published in The Journal of Pharmacology and Experimental Therapeutics. Volume 223, Issue 2, Pages 516–23. PMID 6290642.
- Shim JY, Welsh WJ, Howlett AC (2003). “Unraveling the CB1 Cannabinoid Receptor: Key Sites for Nonclassical Cannabinoid Agonist Interaction.” Published in Biopolymers. Volume 71, Issue 2, Pages 169–89. doi:10.1002/bip.10424. PMID 12767117. S2CID 7909397.
- Roger Pertwee. “Cannabinoids.” Found in the Handbook of Experimental Pharmacology, Volume 168, published by Springer. ISBN 3-540-22565-X.
- Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR (December 1988). “Pharmacological and Stereoselective Characteristics of Novel Cannabinoids in Mouse Models.” Published in The Journal of Pharmacology and Experimental Therapeutics. Volume 247, Issue 3, Pages 1046–51. PMID 2849657.
- “Identification of the Primary Compound in ‘Spice’.” Source: University of Freiburg. [URL: http://www.pr.uni-freiburg.de/pm/2009/pm.2009-01-19.19/]
- “Warning Regarding Smoke Mixtures.” Bundesamt für Sicherheit im Gesundheitswesen (Federal Office of Health Safety) (in German). Dated 16 September 2009.
- Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Ferreirós N (May 2009). “‘Spice’ and Other Herbal Blends: Innocent Incense or Designer Drugs with Cannabinoid Properties?”. Published in the Journal of Mass Spectrometry. Volume 44, Issue 5, Pages 832–7. doi:10.1002/jms.1558. PMID 19189348.
- Compton DR, Johnson MR, Melvin LS, Martin BR (January 1992). “Pharmacological Profile of a Series of Bicyclic Cannabinoid Analogs: Classification as Cannabinoid Mimetics.” Published in The Journal of Pharmacology and Experimental Therapeutics. Volume 260, Issue 1, Pages 201–9. PMID 1309872.
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, et al. (November 1991). “Evaluating Cannabinoid Analogs: Behavioral, Biochemical, and Molecular Modeling Studies.” Published in Pharmacology, Biochemistry, and Behavior. Volume 40, Issue 3, Pages 471–8. doi:10.1016/0091-3057(91)90349-7. PMID 1666911. S2CID 19386120.
- Uchiyama N, Kikura-Hanajiri R, Matsumoto N, Huang ZL, Goda Y, Urade Y (February 2012). “Impact of Synthetic Cannabinoids on Rat EEG Power Spectra.” Published in Forensic Science International. Volume 215, Issues 1–3, Pages 179–83. doi:10.1016/j.forsciint.2011.05.005. PMID 21640532.
- Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y (May 2010). “Chemical Analysis of Synthetic Cannabinoids as Designer Drugs in Herbal Products.” Published in Forensic Science International. Volume 198, Issues 1–3, Pages 31–8. doi:10.1016/j.forsciint.2010.01.004. PMID 20117892.
- “Bundesgetsetsplatz” (PDF). Dated 21 January 2009.
- BGBl I Nr. 3 vom 21.01.2009, 22. BtMÄndV vom 19. Januar 2009, S. 49–50.
- “Version électronique authentifiée publiée au JO n° 0049 du 27/02/2009 – Legifrance.” [URL: www.legifrance.gouv.fr]
- “Grozījumi Ministru kabineta 2005.gada 8.novembra noteikumos Nr.847.” (Amendments to the Cabinet of Ministers Regulations of 8 November 2005, No. 847 “Regulations on Controlled Narcotic Substances, Psychotropic Substances, and Precursors in Latvia”). [URL: LIKUMI.LV]
- “On the Approval of Lists of Narcotic Drugs and Psychotropic Substances.” Issued by the Minister of Health of the Republic of Lithuania.
- Cook M (2011-02-28). “Synthetic Marijuana Banned as of Tuesday.” Published in North County Times, San Diego. Archived from the original on 2011-03-03. Retrieved 2011-02-28.
- “Schedules of Controlled Substances: Temporary Placement of Five Synthetic Cannabinoids Into Schedule I.” Published in the Federal Register on 1 March 2011.