Beautiful Plants For Your Interior
Summary
ADB-FUBINACA, a synthetic designer drug, initially surfaced in synthetic cannabis mixtures in Japan back in 2013. Notably, by 2018, it had risen to become the third most frequently detected synthetic cannabinoid in drug seizures by the Drug Enforcement Administration.
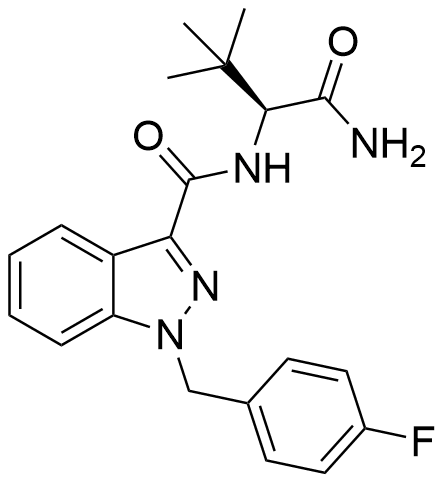
The (S)-enantiomer of ADB-FUBINACA was detailed in a Pfizer patent dating back to 2009. This enantiomer has been recognized for its potent agonistic activity at the CB1 and CB2 receptors, boasting EC50 values of 1.2 nM and 3.5 nM, respectively. ADB-FUBINACA’s structural hallmark is the carboxamide group positioned at the 3-indazole site, akin to SDB-001 and STS-135. Impressively, ADB-FUBINACA results from deliberate and rational drug design, with its primary distinction from AB-FUBINACA lying in the replacement of the isopropyl group with a tert-butyl group.
A related analog, ADSB-FUB-187, has been recently reported. It features a more complex carboxamide substituent, adding to the expanding landscape of synthetic cannabinoids.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1185282-00-1 |
|---|---|
| PubChem CID | 70969086 |
| ChemSpider | 29763706 |
| UNII | 05235E1S2O |
| CompTox Dashboard (EPA) | DTXSID701009981 |
| Chemical and physical data | |
| Formula | C21H23FN4O2 |
| Molar mass | 382.439 g·mol−1 |
Side effects
A single fatality attributed to ADB-FUBINACA intoxication has been connected to coronary arterial thrombosis.
Metabolism
Extensive research has identified twenty-three major metabolites of ADB-FUBINACA in various incubations employing cryopreserved human hepatocytes. Key metabolic pathways involve alkyl and indazole hydroxylation, terminal amide hydrolysis, glucuronide conjugations, and dehydrogenation.
Legality
In the United States, ADB-FUBINACA is categorized as a Schedule I controlled substance.
FAQ
1. What is ADB-FUBINACA?
- ADB-FUBINACA is a synthetic designer drug that is part of the synthetic cannabinoid family. It’s designed to mimic the effects of natural cannabinoids found in cannabis.
2. How did ADB-FUBINACA gain attention?
- ADB-FUBINACA first came into prominence when it was detected in synthetic cannabis blends, particularly in Japan, in 2013. It has since been found in various synthetic drug seizures.
3. What are the known health risks associated with ADB-FUBINACA use?
- ADB-FUBINACA use has been linked to several health risks, including cases of fatalities and adverse reactions. These can range from cardiovascular issues to neurological effects.
4. Is ADB-FUBINACA legal?
- The legality of ADB-FUBINACA varies by country and jurisdiction. In the United States, it is classified as a Schedule I controlled substance, which makes it illegal.
5. Can ADB-FUBINACA be detected in drug tests?
- Yes, ADB-FUBINACA can be detected in drug tests designed to identify synthetic cannabinoids. These tests are becoming more advanced in detecting these substances.
6. What is the metabolic process of ADB-FUBINACA in the human body?
- Studies have identified numerous major metabolites of ADB-FUBINACA in human hepatocytes. The metabolism involves various processes such as hydroxylation, amide hydrolysis, glucuronide conjugations, and dehydrogenation.
7. Are there analogs or related compounds to ADB-FUBINACA?
- Yes, there are analogs and related compounds, as is common in synthetic cannabinoids. Researchers have reported on substances that share similarities with ADB-FUBINACA.
8. What are the legal implications of ADB-FUBINACA possession or distribution?
- The legal implications depend on your location. In the United States, it is illegal and classified as a Schedule I controlled substance. Penalties for possession, distribution, or trafficking can be severe.
9. How can I seek help if I or someone I know is using ADB-FUBINACA?
- Suppose you or someone you know is using ADB-FUBINACA and needs assistance. In that case, it’s important to seek help from a medical professional, addiction counselor, or a local substance abuse support group.
10. Is ADB-FUBINACA related to natural cannabis?
- ADB-FUBINACA is a synthetic compound designed to mimic the effects of natural cannabinoids, but it is not derived from the cannabis plant. It is a product of synthetic drug design.
References
- Anvisa, on July 24, 2023, released “RDC Nº 804,” titled “Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control” in Brazilian Portuguese. This official document was archived from the original on August 27, 2023, and accessed on the same date.
- Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, and Goda Y conducted research in July 2013, unveiling two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids. Their findings were reported in “Forensic Toxicology” (Volume 31, Issue 2) via DOI: 10.1007/s11419-013-0182-9 and S2CID 1279637.
- An overview of synthetic cannabinoids ADB-FUBINACA and AMB-FUBINACA, including clinical, analytical, and forensic implications, was presented by Lobato-Freitas C, Brito-da-Costa AM, Dinis-Oliveira RJ, and others in February 2021. This research is published in “Pharmaceuticals” (Volume 14, Issue 3) and can be accessed through DOI: 10.3390/ph14030186, PMC 7996508, and PMID 33669071.
- The “Emerging Threat Report: Annual 2018,” published by the Special Testing and Research Laboratory, Drug Enforcement Administration, provides insights into emerging threats related to synthetic cannabinoids. This report is available in PDF format.
- WO 2009106982 pertains to “Indazole Derivatives” and is relevant to the subject matter.
- Banister SD, Moir M, Stuart J, and others, in their September 2015 research, delved into the pharmacology of indole and indazole synthetic cannabinoid designer drugs, including AB-FUBINACA, ADB-FUBINACA, and others. This study is accessible through DOI: 10.1021/acschemneuro.5b00112 and PMID 26134475.
- Shanks KG, Clark W, and Behonick G documented a death associated with the use of the synthetic cannabinoid ADB-FUBINACA in April 2016. Their findings can be reviewed in the “Journal of Analytical Toxicology” (Volume 40, Issue 3) via DOI: 10.1093/jat/bkv142, PMC 4885918, and PMID 26755539.
- Carlier J, Diao X, Wohlfarth A, Scheidweiler K, and Huestis MA contributed to the in vitro metabolite profiling of ADB-FUBINACA, a new synthetic cannabinoid, in July 2017. This research can be explored in “Current Neuropharmacology” (Volume 15, Issue 5) via DOI: 10.2174/1570159X15666161108123419, PMC 5771045, and PMID 29403341.
- The “Schedules of Controlled Substances” document released by the Drug Enforcement Administration pertains to the temporary placement of six synthetic cannabinoids, including ADB-FUBINACA, into Schedule I. This document is available through the DEA archives.