Beautiful Plants For Your Interior
Summary
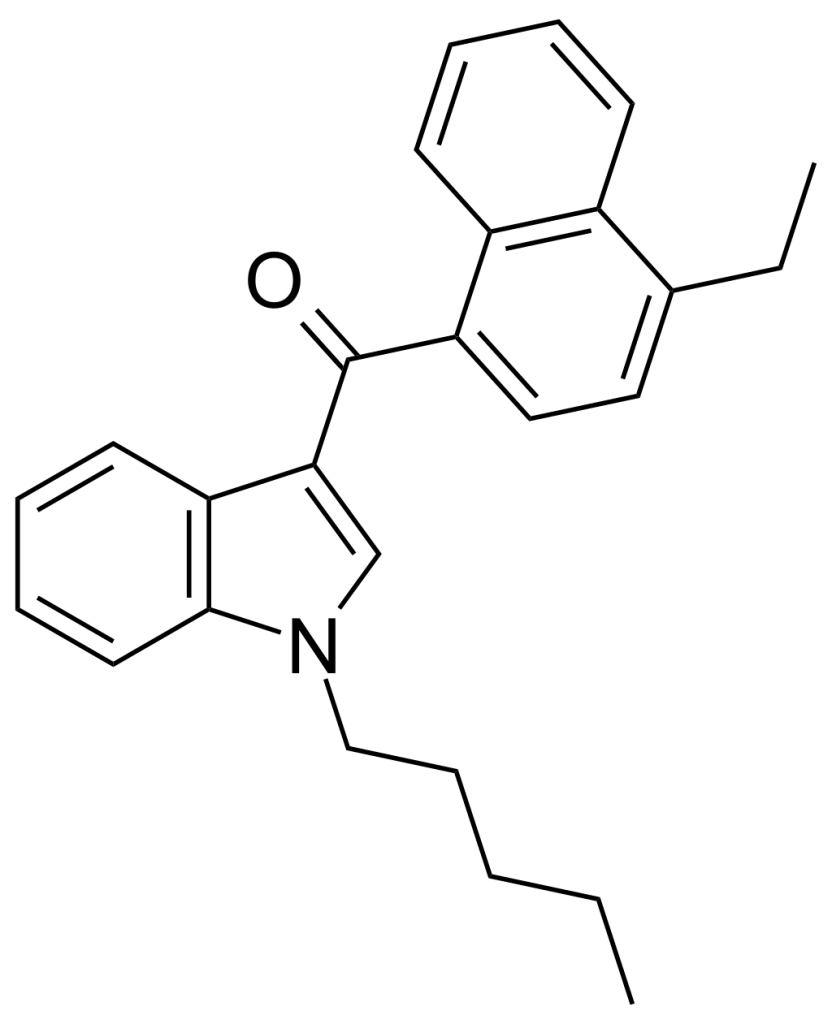
JWH-210, a member of the naphthoylindole family, is a potent analgesic compound. It is a robust cannabinoid agonist, acting on CB1 and CB2 receptors. It demonstrates a remarkable binding affinity with Ki values of 0.46 nM at CB1 and 0.69 nM at CB2. Notably, JWH-210 ranks among the most potent 4-substituted naphthoyl derivatives within the naphthoylindole series, surpassing its 4-methyl and 4-n-propyl counterparts, such as JWH-122 (CB1 Ki 0.69 nM) and JWH-182 (CB1 Ki 0.65 nM). It also exhibits superior binding affinity to the 4-methoxy compound JWH-081 (CB1 Ki 1.2 nM). This chemical was first discovered by and named after John W. Huffman.
It’s worth noting that high doses of JWH-210 when administered to animals, have been associated with potential neurotoxic effects.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 824959-81-1 (JWH-210) 824960-02-3 (JWH-182) |
|---|---|
| PubChem CID | 45270396 |
| ChemSpider | 24617616 |
| UNII | R18JYO04PY |
| CompTox Dashboard (EPA) | DTXSID301010019 |
| ECHA InfoCard | 100.233.380 |
| Chemical and physical data | |
| Formula | C26H27NO |
| Molar mass | 369.508 g·mol−1 |
Legal status
In the United States, all substances falling under the 3-(1-naphthyl)indole class and acting as CB1 receptor agonists, including JWH-210, are categorized as Schedule I Controlled Substances.
Sweden banned JWH-210 and JWH-122 on October 1, 2010, designating them as hazardous substances detrimental to health. This decision followed their identification as components in “herbal” synthetic cannabis products. Subsequently, on September 1, 2011, the Swedish government classified JWH-210, JWH-122, and JWH-203 as illegal drugs.
Furthermore, as of October 2015, JWH-210 is subject to control as a regulated substance in China.
FAQ
1. What is JWH-210?
JWH-210 is a chemical compound classified within the 3-(1-naphthyl)indole class. It acts as a potent agonist for the CB1 receptor and the CB2 receptor and is recognized for its analgesic properties.
2. Is JWH-210 legal in the United States?
No, JWH-210, along with other CB1 receptor agonists of the 3-(1-naphthyl)indole class, is classified as a Schedule I Controlled Substance in the United States, rendering it illegal under federal law.
3. Why were JWH-210 and JWH-122 banned in Sweden?
JWH-210 and JWH-122 were banned in Sweden due to their identification as hazardous substances harmful to health. They were found as components in “herbal” synthetic cannabis products. As a result, the Swedish government classified these substances as illegal drugs.
4. What are the potential effects of JWH-210?
JWH-210 is primarily known for its analgesic properties. As a synthetic cannabinoid, it may produce effects similar to natural cannabinoids, including altered mood, relaxation, and pain relief. However, its use is associated with unpredictable and potentially harmful side effects.
5. Is JWH-210 used for medical purposes?
JWH-210 has not been approved for medical use. Its safety and efficacy for medical applications have yet to be established through rigorous testing. For medical conditions, it is crucial to consult healthcare professionals and use approved medications.
6. Are there risks associated with JWH-210 use?
Yes, using JWH-210 poses risks, including potential health risks and legal consequences. Synthetic cannabinoids, including JWH-210, can lead to unpredictable and harmful side effects, making them unsafe and discouraged.
7. Is JWH-210 regulated in China?
Yes, as of October 2015, JWH-210 is regulated as a controlled substance in China. This indicates that its use may be restricted or prohibited in the country.
8. Where can I find more information about JWH-210?
To learn more about JWH-210, it is advisable to consult reputable scientific literature and healthcare professionals. Always prioritize safety and well-being and adhere to local laws and regulations regarding controlled substances.
References
- Anvisa’s Regulatory Control (2023-07-24): Anvisa, the Brazilian Health Regulatory Agency, issued RDC Nº 804, which outlines a list of substances under special control in Brazilian Portuguese. This list encompasses narcotic, psychotropic, precursor, and other substances. The resolution was published in the Diário Oficial da União on July 25, 2023, and archived for reference. This regulatory update is significant in terms of controlling specific substances in Brazil.
- Polish Amendment Against Drug Abuse (June 2011): The Polish amendment, dated June 2011, introduced changes to the law combating drug abuse. This legislative action addresses issues related to drug control and substance abuse prevention in Poland.
- Pioneering Cannabinoid Receptor Research (January 2005): In a groundbreaking study conducted by Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, and colleagues in January 2005, the structure-activity relationships of 1-alkyl-3-(1-naphthoyl)indoles were explored concerning cannabinoid CB(1) and CB(2) receptors. This research delved into the steric and electronic effects of naphthoyl substituents and paved the way for the development of novel highly selective CB(2) receptor agonists. These findings significantly contributed to our understanding of synthetic cannabinoids and their interactions with specific receptors.
- Neurotoxicity of Synthetic Cannabinoids (November 2015): A study conducted by Cha HJ, Seong YH, Song MJ, Jeong HS, Shin J, Yun J, and others in November 2015 delves into the neurotoxicity of synthetic cannabinoids, specifically JWH-081 and JWH-210. This research explores potential health risks associated with these synthetic compounds, offering valuable insights into their effects.
- United States Controlled Substances Act (21 U.S.C. § 812): The United States Controlled Substances Act, as outlined in Section 21 U.S.C. § 812, establishes and categorizes controlled substances in the United States. This legislative framework is pivotal for regulating various substances, particularly those with the potential for psychoactive effects and misuse, ensuring legal control and safety.
- Swedish Legislation (2010:1086): Swedish Code of Statutes Regulation (2010:1086) is a crucial piece of legislation concerning controlled substances in Sweden. It plays a significant role in the regulation and control of specific substances within the country.
- Chinese Regulations (September 2015): In September 2015, the China Food and Drug Administration issued a notice regarding the management of non-medical narcotic and psychotropic substances. This highlights China’s regulatory measures to control these substances to ensure safety and compliance.