Beautiful Plants For Your Interior
Summary
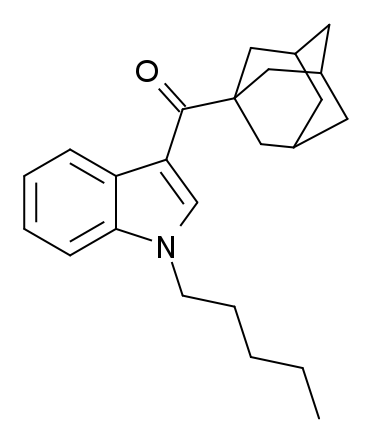
AB-001, also known as 1-pentyl-3-(1-adamantyl)indole, is a designer drug that emerged as an ingredient in synthetic cannabis smoking blends in 2010 in Ireland and 2011 in Hungary and Germany. The original developer of AB-001 remains uncertain; however, it shares structural similarities with compounds like AM-1248 and its corresponding 1-(tetrahydropyran-4-ylmethyl) analogue, which are recognized as potent cannabinoid agonists with moderate to high selectivity for CB2 over CB1.
The first published synthesis and pharmacological assessment of AB-001 revealed its complete agonist properties at CB1 (EC50 = 35 nM) and CB2 receptors (EC50 = 48 nM). Interestingly, despite its potent binding affinity, AB-001 exhibited relatively weak cannabimimetic effects in rats, even at doses as high as 30 mg/kg. This contrasts with the carboxamide analogue APICA, which demonstrates robust cannabimimetic activity at much lower amounts of 3 mg/kg.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1345973-49-0 |
|---|---|
| PubChem CID | 57404070 |
| ChemSpider | 26286891 |
| UNII | H657DIA015 |
| CompTox Dashboard (EPA) | DTXSID90158822 |
| Chemical and physical data | |
| Formula | C24H31NO |
| Molar mass | 349.518 g·mol−1 |
FAQ
- What is AB-001, and how did it come to public attention?AB-001, also known as 1-pentyl-3-(1-adamantoyl)indole, is a designer drug that was identified as an ingredient in synthetic cannabis smoking blends. It gained attention after being found in these products in Ireland in 2010 and later in Hungary and Germany in 2011.
- Who originally developed AB-001, and what are its structural similarities to other compounds?The exact origin of AB-001 remains unclear. However, it shares structural similarities with compounds like AM-1248 and its corresponding 1-(tetrahydropyran-4-ylmethyl) analogue. These related compounds are known for their potency as cannabinoid agonists with varying selectivity for CB2 over CB1 receptors.
- What is the pharmacological profile of AB-001, and how does it interact with cannabinoid receptors?Research has shown that AB-001 acts as a full agonist at both CB1 and CB2 receptors, with EC50 values of 35 nM and 48 Nm, respectively. This indicates its ability to bind to and activate these receptors.
- What are the effects of AB-001 when tested in animal models?Interestingly, despite its potent binding affinity to cannabinoid receptors, AB-001 exhibited only weak cannabimimetic effects in rats, even at relatively high doses of up to 30 mg/kg. This makes it less potent in producing cannabimimetic effects compared to other related compounds.
- How does the effectiveness of AB-001 compare to the carboxamide analogue APICA?AB-001 is less potent in terms of cannabimimetic activity in rats compared to the carboxamide analogue APICA. While AB-001 requires higher doses to produce effects, APICA demonstrates strong cannabimimetic activity at much lower doses.
- Is AB-001 legal, and where is it regulated or controlled?The legal status of AB-001 varies by location. It’s essential to consult local laws and regulations to determine its status in a specific region.
- Where can I find more information about AB-001 and related designer drugs?For comprehensive information on AB-001 and similar designer drugs, consider referring to scientific publications, academic sources, and regulatory authorities. Always prioritize safety and compliance with relevant laws when dealing with such substances.
References
- Jankovics P, Váradi A, Tölgyesi L, Lohner S, Németh-Palotás J, Balla J. “Detection and identification of the new potential synthetic cannabinoids 1-pentyl-3-(2-iodobenzoyl)indole and 1-pentyl-3-(1-adamantoyl)indole in seized bulk powders in Hungary.” (Forensic Science International. 2012; 214(1–3): 27–32).
- Research on Head Shop drugs in Dublin: Part 2.
- Grigoryev A, Kavanagh P, Melnik A. “The detection of the urinary metabolites of 3-[(adamantan-1-yl)carbonyl]-1-pentylindole (AB-001), a novel cannabimimetic, by gas chromatography-mass spectrometry.” (Drug Testing and Analysis. 2012; 4(6): 519–24).
- US patent 7820144, Makriyannis A, Deng H. “Receptor selective cannabimimetic aminoalkylindoles,” granted on October 26, 2010.
- Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, et al. “Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity.” (Journal of Medicinal Chemistry. 2010; 53(1): 295–315).
- Banister SD, Wilkinson SM, Longworth M, Stuart J, Apetz N, English K, et al. “The synthesis and pharmacological evaluation of adamantane-derived indoles: cannabimimetic drugs of abuse.” (ACS Chemical Neuroscience. 2013; 4(7): 1081–92).