Beautiful Plants For Your Interior
Summary
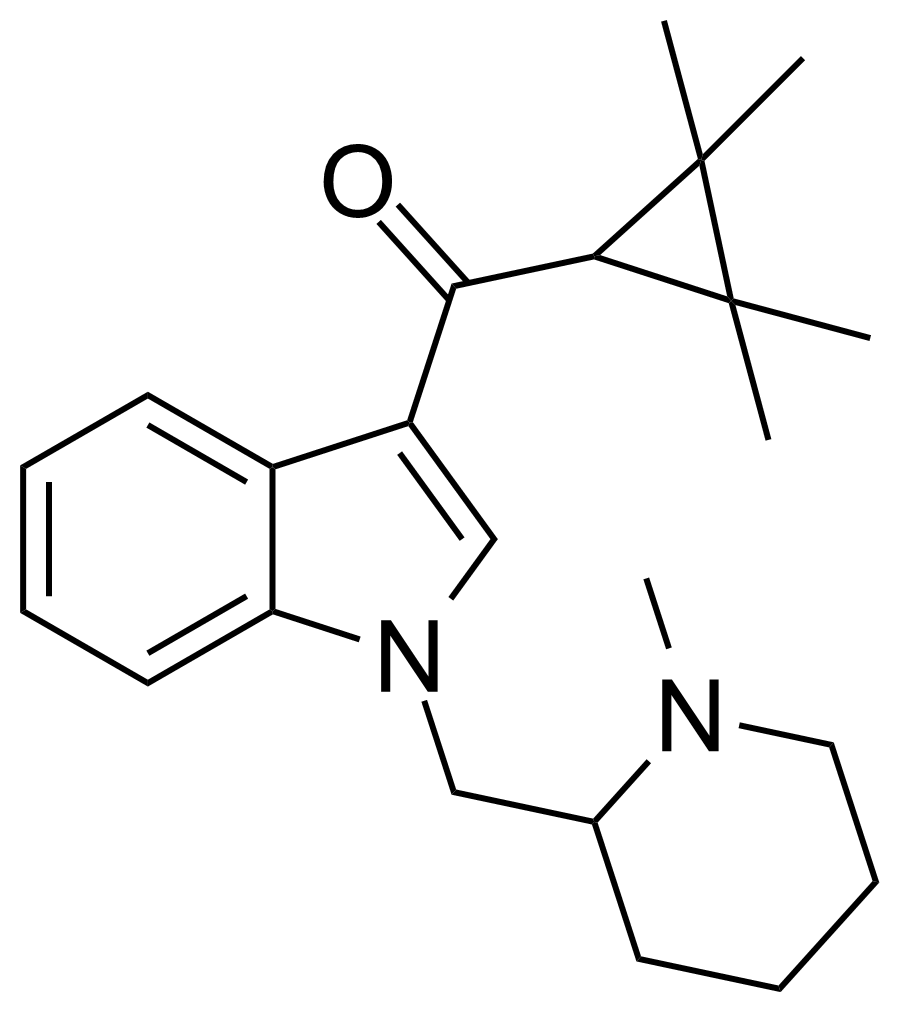
AB-005, also known as 1-[(1-methylpiperidin-2-yl)methyl]-1H-indol-3-yl-methanone, is a designer drug offered by online vendors, positioned as a cannabimimetic agent. This compound’s structure and pharmacological activity were first documented in 2010, well before its availability in the commercial market in 2012. Studies revealed that AB-005 exhibits a notable affinity for CB1 receptors (Ki = 5.5 nM) and CB2 receptors (Ki = 0.48 nM), underscoring its potential as a potent activator of these cannabinoid receptors.
AB-005 shares structural features with previously reported synthetic cannabinoids, notably incorporating the tetramethylcyclopropane group in UR-144 and XLR-11. Additionally, it features the (1-methyl-2-piperidinyl)methyl substituent, akin to compounds like AM-1248 and AM-1220. Despite these insights into its structure and receptor affinity, the in vivo effects of AB-005 remain largely unexplored.
To date, there is a shortage of published information concerning the in vivo activity of AB-005. Consequently, our understanding of its effects on humans is primarily based on anecdotal reports, and further research is warranted to assess its psychoactivity and potential health implications comprehensively.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 895155-25-6 |
|---|---|
| PubChem CID | 11537652 |
| ChemSpider | 9712433 |
| UNII | TPY5M47AYQ |
| ChEMBL | ChEMBL571330 |
| Chemical and physical data | |
| Formula | C23H32N2O |
| Molar mass | 352.522 g·mol−1 |
Legal status
In 2013, New Zealand granted provisional approval to psychoactive products containing this substance, in accordance with psychoactive substances legislation.
FAQ
1. What is AB-005?
- AB-005, also known by its chemical name 1-[(1-methylpiperidin-2-yl)methyl]-1H-indol-3-yl-methanone, is a designer drug available from online vendors. It is marketed as a cannabimimetic agent.
2. When was AB-005’s structure and pharmacological activity first documented?
- AB-005’s structure and pharmacological activity were reported in 2010, several years before its commercial availability in 2012.
3. What is AB-005’s affinity for cannabinoid receptors?
- AB-005 has a high affinity for CB1 and CB2 receptors, with a Ki (inhibition constant) of 5.5 nM for CB1 and 0.48 nM for CB2 receptors. This suggests that it is a potent activator of these cannabinoid receptors.
4. Are there structural similarities between AB-005 and other synthetic cannabinoids?
- Yes, AB-005 shares structural elements with previously known synthetic cannabinoids. It includes the tetramethylcyclopropane group found in compounds like UR-144 and XLR-11. Additionally, it features the (1-methyl-2-piperidinyl)methyl substituent, which is similar to compounds like AM-1248 and AM-1220.
5. What do we know about the in vivo activity of AB-005?
- Very little information is available about the in vivo activity of AB-005. To date, no comprehensive studies have been published regarding its effects on living organisms.
6. How is AB-005 regulated?
- The legal status and regulation of AB-005 vary by jurisdiction. It’s crucial to be aware of the specific laws and regulations in your region regarding the possession, use, and distribution of designer drugs like AB-005.
7. Is AB-005 safe for consumption?
- The safety of AB-005 has not been extensively studied, and its potential health risks are largely unknown. As with any unregulated designer drug, caution is advised, and its consumption is discouraged due to the lack of safety data.
8. Where can I find more information about AB-005 and its effects?
- For comprehensive information about AB-005, including its chemical properties, potential risks, and legal status, it is advisable to consult credible sources such as government health agencies, medical professionals, and substance abuse organizations.
References
- Effects of N1 Substituted Indole Side Chain Variations on CB2 Cannabinoid Receptor Activity – Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, et al. (January 2010). This study delves into the impact of N1 substituted indole side chain variations on CB2 cannabinoid receptor activity, shedding light on the pharmacological aspects of substances like AB-005.
- Interim Product Approvals – Ministry of Health. This official source provides information about the interim approval of products containing AB-005 in New Zealand in 2013, in accordance with psychoactive substances legislation. It offers insights into the regulatory status of this compound in a specific region.