Beautiful Plants For Your Interior
Summary
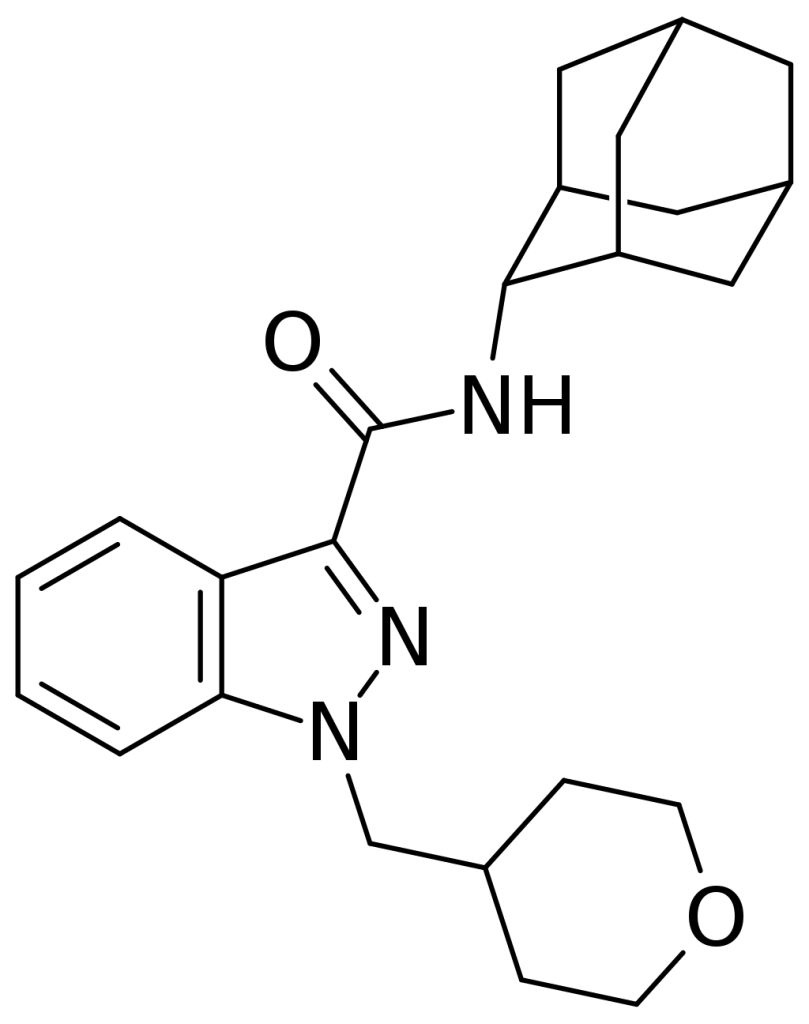
Adamantyl-THPINACA, often abbreviated as ATHPINACA or AD-THPINACA, is a synthetic cannabinoid compound based on the indazole structure. Its presence was initially documented in Slovenia in January 2015, and it exists in two distinct isomeric forms: the 1-adamantyl and 2-adamantyl isomers, identified as SGT-40 and SGT-194, respectively, a differentiation that can be established through GC-EI-MS analysis.
This synthetic substance is subject to legal restrictions in several countries. Notably, Sweden and Russia have prohibited its use and possession. Additionally, both the 1-adamantyl and 2-adamantyl isomers are explicitly classified as illegal drugs in Japan.
One interesting aspect of Adamantyl-THPINACA is its potential to release amantadine through metabolic processes, a phenomenon suspected due to the known metabolic liberation of amantadine in a related compound called APINACA, in which amantadine appears as an impurity. This suggests that metabolic hydrolysis of the amide group in Adamantyl-THPINACA might also yield amantadine.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 2365471-86-7 1400742-48-4 (1-adamantyl isomer) |
|---|---|
| PubChem CID | 123132009 |
| ChemSpider | 58858889 |
| UNII | 7I4LAF1R3Y |
| CompTox Dashboard (EPA) | DTXSID101009995 |
| Chemical and physical data | |
| Formula | C24H31N3O2 |
| Molar mass | 393.531 g·mol−1 |
FAQ
1. What is Adamantyl-THPINACA (ATHPINACA)?
- Adamantyl-THPINACA, also known as ATHPINACA or AD-THPINACA, is a synthetic cannabinoid compound based on the indazole structure. It is associated with the family of designer drugs and has raised concerns due to its psychoactive effects.
2. How was ATHPINACA first discovered?
- ATHPINACA was first reported to Europol in Slovenia in January 2015. This marked its initial recognition as a synthetic cannabinoid, and it has since garnered attention from regulatory authorities and researchers.
3. What are the different isomeric forms of ATHPINACA?
- ATHPINACA exists in two distinct isomeric forms: the 1-adamantyl and 2-adamantyl isomers. These are referred to as SGT-40 and SGT-194, respectively. They can be differentiated through gas chromatography-electron ionization mass spectrometry (GC-EI-MS) analysis.
4. In which countries is ATHPINACA banned or restricted?
- ATHPINACA is subject to legal restrictions in various countries. It is prohibited in Sweden and Russia. Japan specifically classifies both the 1-adamantyl and 2-adamantyl isomers as illegal drugs, making their possession and use unlawful.
5. What is the significance of amantadine in ATHPINACA?
- Amantadine is suspected to be released through metabolic processes in the presence of ATHPINACA. This suspicion is based on the known metabolic liberation of amantadine in a related compound called APINACA, where amantadine appears as an impurity. Researchers believe metabolic hydrolysis of the amide group in ATHPINACA may yield amantadine.
6. Is ATHPINACA safe for use?
- Synthetic cannabinoids like ATHPINACA have not been thoroughly studied for their safety and long-term effects. These substances can be associated with various health risks, and their use is generally discouraged due to the lack of regulatory oversight.
7. Are there any medical or therapeutic applications for ATHPINACA?
- As of my knowledge, the cutoff date is January 2022; ATHPINACA is not approved for any medical or therapeutic use. It is primarily encountered in the context of recreational drug use and has not undergone clinical trials or evaluations for medical purposes.
References
- “ATHPINACA isomer 1 (CAS 1400742-48-4)” was documented by Caymen Chemical and can be traced back to an entry from 29th April 2017.
- A report titled “Muuntohuumeiden esiintyvyys 2015” in PDF format was published by the Finnish Medicines Agency on 7th May 2015, and it was retrieved on 10th March 2021.
- The “Europol 2015 Annual Report” focuses on the execution of Council Decision 2005/387/JHA.
- In a research conducted by Asada A, Doi T, Tagami T, Takeda A, and Sawabe Y in March 2017, they discuss the differentiation of synthetic cannabinoids through GC-EI-MS, particularly the 1-adamantyl and 2-adamantyl isomers of N-adamantyl carboxamides. This study has been documented in “Drug Testing and Analysis” (Volume 9, Issue 3) and holds a DOI of 10.1002/dta.2124, with a PMID of 27770510.
- The “Fler ämnen föreslås bli klassade som narkotika eller hälsofarlig vara” is an article retrieved from Folkhälsomyndigheten on 24th July 2015.
- The “List of substances prohibited in the Russian Federation” is another notable reference.
- Lastly, the “指定薬物一覧” is a list of designated drugs that holds significance in a specific context.