Beautiful Plants For Your Interior
Summary
ADB-FUBIATA, also known as AD-18 or FUB-ACADB, emerged as a synthetic cannabinoid compound in 2021. It shares structural similarities with the older combination ADB-FUBICA, with a notable distinction – an extended amide linker group by adding a methylene bridge. This compound found its way into the market as an ingredient in grey-market synthetic cannabis blends. This shift was prompted by changes in Chinese legislation, which introduced comprehensive controls on various classes of synthetic cannabinoids but did not encompass compounds with the specific linker group extension.
ADB-FUBIATA exhibits significantly lower affinity for cannabinoid receptors than ADB-FUBICA, boasting an EC50 of only 635 nM at CB1. However, it still acts as a full agonist at the CB1 receptor while demonstrating negligible activity at CB2.
| Identifiers | |
|---|---|
| IUPAC name | |
| PubChem CID | 165361558 |
|---|---|
| ChemSpider | 114876989 |
| Chemical and physical data | |
| Formula | C23H26FN3O2 |
| Molar mass | 395.478 g·mol−1 |
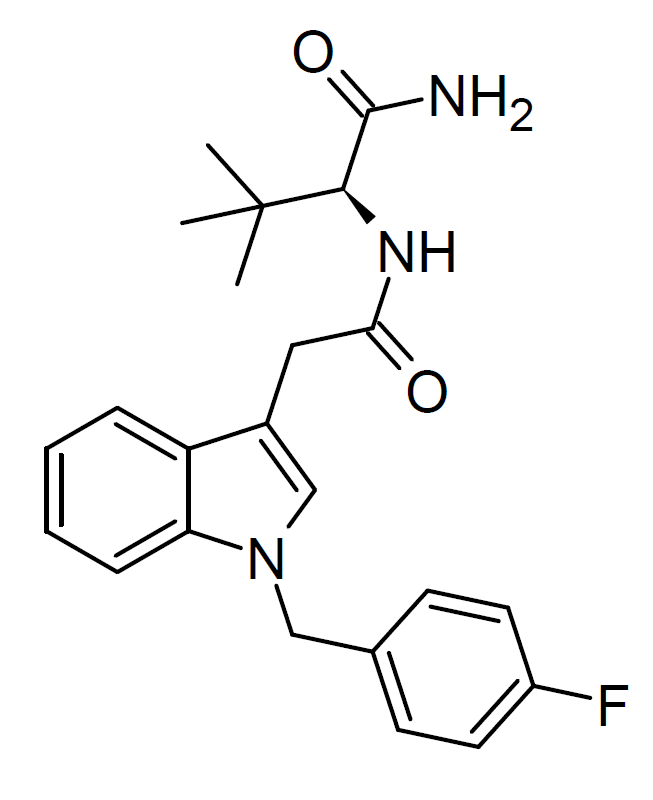
Legality
As of May 22, 2023, ADB-FUBIATA remains unscheduled at the federal level in the United States. However, it could fall under the purview of the federal analogue act if marketed for human consumption. Notably, North Dakota took the step of classifying ADB-FUBIATA as a Schedule I substance on April 27, 2023.
fAQ
1. What is ADB-FUBIATA?
ADB-FUBIATA is a synthetic cannabinoid compound that emerged in 2021. It shares structural similarities with ADB-FUBICA but features an extended amide linker group.
2. Is ADB-FUBIATA legal in the United States?
As of May 22, 2023, ADB-FUBIATA is unscheduled at the federal level in the United States. However, it may be regulated under the federal Analogue Act if marketed for human consumption.
3. What is the Federal Analogue Act?
The Federal Analogue Act allows the U.S. government to regulate substances that are chemically similar to controlled substances, even if they are not explicitly listed as controlled substances.
4. What is the legal status of ADB-FUBIATA in North Dakota?
North Dakota has classified ADB-FUBIATA as a Schedule I substance as of April 27, 2023. This means it is considered a controlled substance with a high potential for abuse and no recognized medical use.
5. What are the potential effects of ADB-FUBIATA?
ADB-FUBIATA’s effects may include altered mood, perception, and relaxation due to its interaction with the endocannabinoid system. However, synthetic cannabinoids can have unpredictable and potentially harmful effects.
6. Is ADB-FUBIATA safe to use?
The safety of ADB-FUBIATA is a subject of concern. Synthetic cannabinoids can have adverse effects, and their safety profile is not well-established. Use with caution and adhere to relevant laws and regulations.
7. Can ADB-FUBIATA be used for medical purposes?
Research on the medical applications of ADB-FUBIATA is limited. It is primarily considered a recreational or designer drug, and its use for medical purposes is not established.
8. What are the risks associated with ADB-FUBIATA use?
Using synthetic cannabinoids like ADB-FUBIATA can pose various risks, including unpredictable effects, adverse reactions, and potential legal consequences. Long-term health effects are still not well understood.
9. Where can I find more information about ADB-FUBIATA?
Seek information from reputable sources, including scientific literature and government websites, to learn more about ADB-FUBIATA and its regulatory status. Consult with healthcare professionals or experts for accurate and up-to-date information.
10. What should I do if I have concerns about ADB-FUBIATA use or addiction?
If you or someone you know is struggling with substance abuse or addiction related to ADB-FUBIATA or other substances, reach out to addiction treatment centres or support groups for guidance, assistance, and support.
References
- Anvisa (2023-07-24) issued “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] on July 24, 2023. This regulatory action was published in the Diário Oficial da União on July 25, 2023, and can be accessed in Brazilian Portuguese. The original document was archived on August 27, 2023, with retrieval taking place on the same date.
- On May 12, 2021, the Ministry of Public Security of the People’s Republic of China issued an announcement titled “关于将合成大麻素类物质和氟胺酮等18种物质列入《非药用类麻醉药品和精神药品管制品种增补目录》的公告” [Announcement on the inclusion of 18 substances including synthetic cannabinoids and fluamine in the “Additional Catalogue of Controlled Varieties of Non-medicinal Narcotics and Psychotropic Drugs”]. This announcement was made in Chinese and pertains to the control of various substances.
- In September 2022, Deventer MH, Van Uytfanghe K, Vinckier IM, Reniero F, Guillou C, and Stove CP introduced a new cannabinoid receptor 1 selective agonist in their research, titled “A new cannabinoid receptor 1 selective agonist evading the 2021 ‘China ban’: ADB-FUBIATA.” Their findings were published in the “Drug Testing and Analysis” journal, Volume 14, Issue 9, spanning pages 1639–1644. The research article can be accessed via doi:10.1002/dta.3285, with the PMID being 35570246. Additionally, the article can be found on S2CID 248812121.
- Pasin D, Nedahl M, Mollerup CB, Tortzen C, Reitzel LA, and Dalsgaard PW identified the synthetic cannabinoid-type new psychoactive substance, CH-PIACA, in seized material in their research published in September 2022. The article, titled “Identification of the synthetic cannabinoid-type new psychoactive substance, CH-PIACA,” was published in the “Drug Testing and Analysis” journal, Volume 14, Issue 9, covering pages 1645–1651. You can access the research via doi:10.1002/dta.3333, and it is available on PMC with the identifier PMC 9544820. The PMID for this publication is 35687099.
- On January 3, 2023, the Sixty-eighth Legislative Assembly of North Dakota in Regular Session issued an act aimed at amending and reenacting sections 19-03.1-05, 19-03.1-11, and 19-03.1-13 of the North Dakota Century Code. This legislative action pertained to the scheduling of controlled substances and declared an emergency. The document is available in PDF format.