Beautiful Plants For Your Interior
- 1 Where to Buy Butyrfentanyl Online – Trusted Vendors and Sources
- 2 How to Order Butyrfentanyl – A Step-by-Step Guide for Safe Purchases
- 3 Butyrfentanyl for Sale – Best Deals and Discounts
- 4 Butyrfentanyl USA – Where to Buy Butyrfentanyl in the United States
- 5 Summary
- 6 Pharmacokinetics
- 7 Legal Status:
- 8 FAQ
- 9 References
Where to Buy Butyrfentanyl Online – Trusted Vendors and Sources
If you’re looking to buy Butyrfentanyl online, it’s crucial to find a trusted vendor. Numerous online shops offer Butyrfentanyl for sale, but selecting a reliable vendor ensures the authenticity and safety of the product. Look for vendors with good customer reviews, secure payment methods, and clear shipping policies. Whether you’re located in the USA, Canada, or internationally, make sure the vendor offers discreet shipping and complies with local regulations.
How to Order Butyrfentanyl – A Step-by-Step Guide for Safe Purchases
Ordering Butyrfentanyl online is simple if you follow the correct steps. Start by choosing a reputable Butyrfentanyl shop that provides secure payment options and discreet shipping. Be sure to verify the vendor’s reputation through customer feedback and product reviews. Once you’ve decided on the quantity, proceed to checkout and complete your purchase securely. Always confirm shipping details and track your order to ensure timely delivery.
Butyrfentanyl for Sale – Best Deals and Discounts
If you’re looking for Butyrfentanyl for sale, you may find promotions or discounts available for bulk purchases. Many trusted vendors offer lower prices for larger orders or provide special offers for first-time buyers. By comparing prices and offers from different Butyrfentanyl vendors, you can secure the best deal while ensuring the product’s quality. Be on the lookout for free shipping or other promotions to further reduce your overall costs.
Butyrfentanyl USA – Where to Buy Butyrfentanyl in the United States
For customers in the USA, buying Butyrfentanyl online is convenient, with several reputable vendors offering shipping within the United States. However, it is essential to check the legal status of Butyrfentanyl in your state, as research chemicals can have varying legal restrictions depending on location. Make sure to purchase from a vendor who complies with US regulations, offers secure payment methods, and guarantees discreet shipping.
Summary
Butyrfentanyl, also known as butyrylfentanyl, is a potent, short-acting synthetic opioid analgesic. It serves as an analog of fentanyl, boasting roughly one-quarter of its potency. The initial reference to this compound can be traced back to a document published by The College on Problems of Drug Dependence, where it is denoted as “N-butyramide fentanyl analog.” This document also indicates that a comprehensive article detailing its clinical effects, encompassing analgesic studies, binding to μ-, δ-, and κ-opioid receptors, as well as in vitro assessments of drug efficacy, antinociceptive properties, and narcotic attributes, was made available in 1987. Butyrfentanyl predominantly acts as an agonist for the μ-opioid receptors.
Presently, fentanyl does not possess any legitimate clinical applications; instead, it is illicitly distributed as a designer drug.
The side effects associated with fentanyl analogs are akin to those of fentanyl itself. They may encompass symptoms such as itching, nausea, and potentially severe respiratory depression, which can pose life-threatening risks. Fentanyl analogs have been linked to numerous fatalities across Europe and the former Soviet republics since their resurgence in usage commenced in Estonia during the early 2000s. Furthermore, new derivatives within this class of compounds continue to emerge.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1169-70-6 |
|---|---|
| PubChem CID | 621174 |
| ChemSpider | 539764 |
| UNII | 07V1H7R6ZN |
| CompTox Dashboard (EPA) | DTXSID30347410 |
| Chemical and physical data | |
| Formula | C23H30N2O |
| Molar mass | 350.506 g·mol−1 |
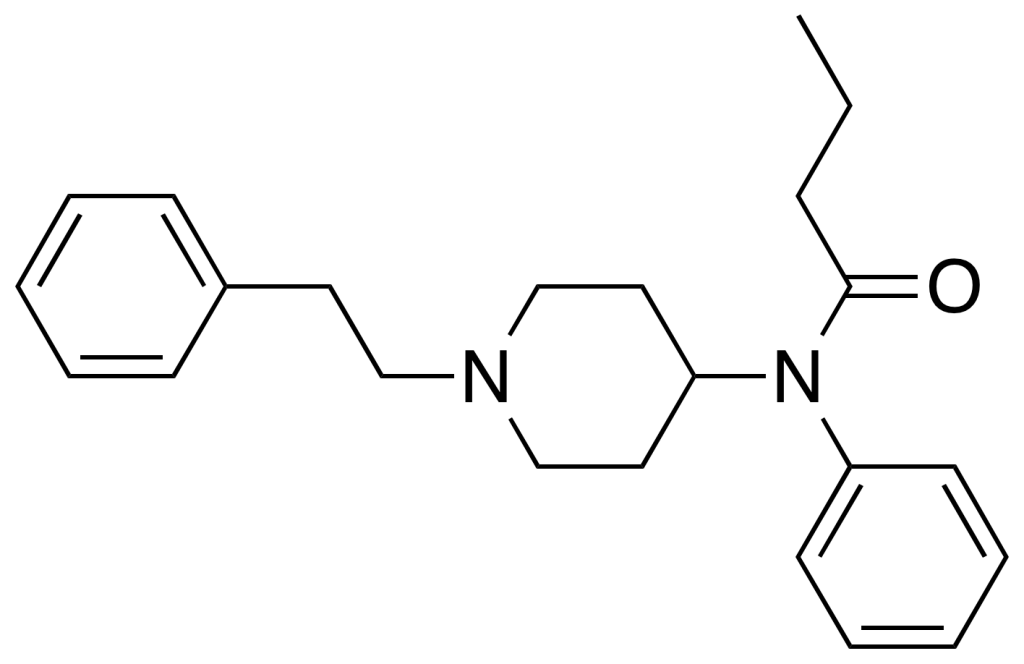
Pharmacokinetics
Butyrfentanyl exhibits an affinity for the opioid receptor. In studies investigating the in vitro inhibition of specific [3H] fentanyl binding to the opioid receptor, the analogs were ranked in the following order: (±)-cis-3-methylfentanyl > fentanyl = alpha-methylfentanyl > butyrylfentanyl > benzylfentanyl. Similar findings were observed in studies examining binding affinity inhibition, reinforcing that butyrfentantyl is a less potent opioid agonist compared to fentanyl. On another note, research into the cross-reactivity with the fentanyl antibody among fentanyl and its analogs indicated the order as: fentanyl = butyrylfentanyl > (±)-cis-3-methylfentanyl > benzylfentanyl > alpha-methylfentanyl. The high cross-reactivity can be attributed to the structural similarity between butyrfentanyl and the original fentanyl molecule, making it susceptible to binding by fentanyl antibodies.
The opioid receptor affinity of butyrfentanyl was assessed based on its inhibitory potency in a binding assay using [3H] fentanyl as the radioligand. The Ki value for butyrfentanyl was Ki=32 ± 4.1 nM. In comparison to fentanyl’s Ki (Ki=1.06 ± 0.15 nM), butyrfentanyl’s ability to displace [3H] fentanyl is notably lower, requiring higher drug concentrations.
Studies on urinary excretion revealed that almost all of the injected butyrfentanyl was excreted or metabolized within the initial 3 hours post-injection, with only deficient concentrations remaining detectable after this period. Urinary concentrations of butyryl fentanyl from animals injected with 15 μg/kg and 45 μg/kg intravenously were measured using two techniques: radioreceptor assay and gas chromatography/mass spectrometry (GC/MS).
Legal Status:
- UN Commission on Narcotic Drugs: The UN Commission on Narcotic Drugs passed a proposal on March 16, 2017, to include butyrfentanyl in Schedule 1 of the 1961 Single Convention on Narcotic Drugs.
- United Kingdom: Butyrfentanyl is illegal in the United Kingdom due to its modification of fentanyl by replacing the N-propionyl group with another acyl group.
- United States: As of May 2016, fentanyl is classified as a Schedule I controlled substance in the United States.
- China: As of October 2015, fentanyl is considered a controlled substance in China.
- Switzerland: Butyrfentanyl has been illegal in Switzerland since December 2015.
FAQ
- What is Butyrfentanyl?
- Butyrfentanyl is a synthetic opioid analgesic drug and an analog of Fentanyl. It is known for its potent pain-relieving effects but is significantly less powerful than Fentanyl itself.
- How does Butyrfentanyl compare to Fentanyl in terms of potency?
- Butyrfentanyl is estimated to be approximately one-quarter as potent as Fentanyl, making it less powerful in terms of its opioid effects.
- Are there legitimate medical uses for Butyrfentanyl?
- No, Butyrfentanyl has no recognized legitimate clinical applications and is not used for medical purposes. It is mainly encountered as a designer drug in illicit contexts.
- What are the side effects associated with Butyrfentanyl use?
- Side effects of Butyrfentanyl and similar fentanyl analogs include itching, nausea, and the potential for severe respiratory depression, especially in cases of overdose or when used in combination with other drugs like benzodiazepines. Respiratory depression can be life-threatening.
- How does Butyrfentanyl’s binding affinity to opioid receptors compare to that of Fentanyl?
- Butyrfentanyl exhibits lower binding affinity to opioid receptors compared to Fentanyl. It is a less potent opioid agonist, requiring higher concentrations to displace Fentanyl from these receptors.
- What is the legal status of Butyrfentanyl in various countries?
- UN Commission on Narcotic Drugs: It was included in Schedule 1 of the 1961 Single Convention on Narcotic Drugs in March 2017.
- United Kingdom: Illegal due to its modification of Fentanyl.
- United States: Classified as a Schedule I controlled substance since May 2016.
- China: Considered a controlled substance as of October 2015.
- Switzerland: It has been illegal in Switzerland since December 2015.
- Is Butyrfentanyl commonly used as a recreational drug?
- Butyrfentanyl is frequently sold and used as a designer drug in illicit or recreational contexts, although it poses significant risks, especially when abused or combined with other substances.
- Is Butyrfentanyl related to any other synthetic opioids?
- Yes, Butyrfentanyl is related to Fentanyl and belongs to the family of synthetic opioids. It shares structural similarities with other analogs and derivatives within this class.
- What is its pharmacological profile compared to other fentanyl analogs?
- Butyrfentanyl’s pharmacological profile is characterized by its lower binding affinity to opioid receptors, which results in reduced potency compared to Fentanyl and some of its analogs.
- How is Butyrfentanyl detected or measured in the body?
- Studies on urinary excretion of Butyrfentanyl have shown that it is excreted or metabolized within the first 3 hours after injection. Researchers use various techniques, including radioreceptor assay and gas chromatography/mass spectrometry (GC/MS), to detect and measure its presence in biological samples.
References
- The College on Problem of Drug Dependence: The organization provided valuable insights into the world of drug dependence through its Foreword to the Drug Evaluation Committee (DEC) Analgesic, Stimulant, and Depressant Drug Indices.
- Utilization of a radioreceptor assay for the analysis of fentanyl analogs in urine: Mario Enrique Alburges’ pioneering work shed light on the analysis of fentanyl analogs in urine samples, utilizing a radioreceptor assay.
- An Acute Butyr-Fentanyl Fatality: In a poignant case report published in the Journal of Analytical Toxicology, Iain M. McIntyre and colleagues examined a fatality involving Butyr-Fentanyl, highlighting postmortem concentrations and its implications.
- Fentanyls: Are we missing the signs?: Jane Mounteney, Isabelle Giraudon, Gleb Denissov, and Paul Griffiths explored the rising prominence of highly potent fentanyls in Europe, raising questions about whether we are overlooking the signs of this emerging issue.
- UN Commission on Narcotic Drugs Decision: The UN Commission on Narcotic Drugs reached a decision to include certain substances, shedding light on global efforts to regulate controlled substances effectively.
- Schedules of Controlled Substances: The U.S. Drug Enforcement Administration documented the temporary placement of Butyryl Fentanyl and Beta-Hydroxythiofentanyl into Schedule I, signifying a significant step in controlling these substances.
- China’s Regulation on Non-Medical Narcotic and Psychotropic Substances: The China Food and Drug Administration issued a crucial notice regarding the regulation of non-medical narcotic and psychotropic substances, emphasizing the importance of effective governance in this realm.
- Switzerland’s Regulation on Narcotic Substances: The Swiss Federal Council issued a regulation pertaining to narcotic substances, underscoring the nation’s commitment to managing these substances and their derivatives.