The market situation for 5-APB, a designer drug and research chemical, has evolved significantly in recent years. Individuals interested in exploring its psychoactive effects often seek this compound. Sellers and vendors, primarily operating online, have been crucial in facilitating its availability.
Online platforms have become the preferred marketplace for buying and selling 5-APB. Vendors offering this research chemical can quickly reach a global customer base, and buyers can access a wide range of options. However, it’s important to note that the legality of 5-APB varies by country, and potential buyers should be aware of local regulations before purchasing.
As with many designer drugs, the market for 5-APB is dynamic and subject to changes in legislation and law enforcement efforts. Some regions have implemented strict controls or outright bans on its sale, impacting its availability.
Despite legal challenges, 5-APB remains in demand among those seeking novel experiences. The market’s resilience highlights the ongoing interest in research chemicals and the adaptability of sellers and buyers within this niche. It is crucial for individuals considering purchasing or using 5-APB to stay informed about legal restrictions and to prioritize safety and responsible use.
- 1 Summary
- 2 History and culture
- 3 Chemistry
- 4 Pharmacology
- 5 Subjective effects
- 6 Toxicity
- 7 Legal status
- 8 FAQ
- 8.1 1. What is 5-APB?
- 8.2 2. What are the effects of 5-APB?
- 8.3 4. Is 5-APB legal?
- 8.4 5. What are the risks associated with 5-APB use?
- 8.5 6. Is 5-APB safe to use?
- 8.6 7. Can 5-APB be tested for or detected in drug screenings?
- 8.7 8. What is the recommended dosage of 5-APB?
- 8.8 9. Can 5-APB be used for therapeutic purposes?
- 8.9 10. What should I do in case of an adverse reaction or overdose?
- 9 References
Summary
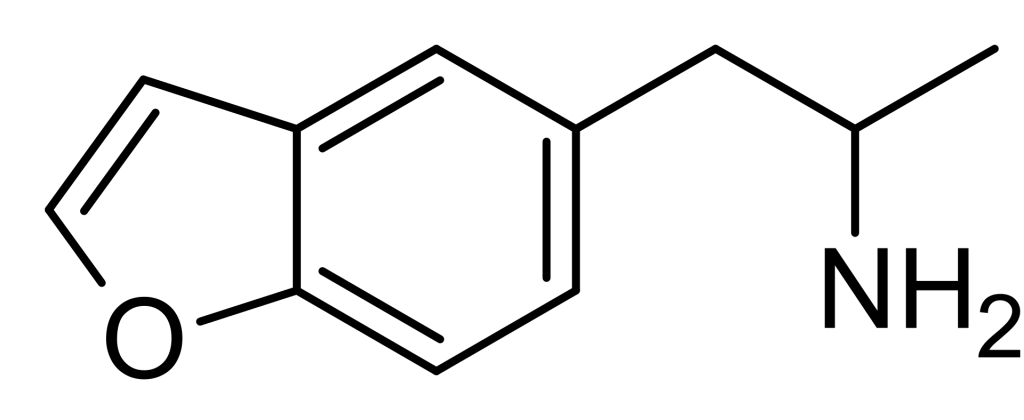
5-(2-Aminopropyl)benzofuran, commonly called 5-APB, belongs to the benzofuran class and is a relatively lesser-known entactogen. When administered, it elicits entactogenic and stimulating effects reminiscent of MDA. Structurally, it shares similarities with other entactogens like 5-MAPB, 6-APB, and MDA.
The synthesis of 5-APB dates back to 1993, when it was created by the pioneering psychedelic chemist and researcher David E. Nichols. Its development aimed to provide a potentially non-neurotoxic alternative to MDMA, although it remained relatively obscure for over a decade. It eventually gained popularity within the rave scene and the global market for research chemicals. Initially, it was marketed under the moniker “Benzofury” alongside other novel benzofuran entactogens before facing bans on its sale and import.
What sets 5-APB apart from its chemical counterparts like 6-APB and 5-MAPB is its reputation for inducing stimulating and euphoric effects. This distinct profile contributed to its ascent in popularity, notably as a readily accessible product through online research chemical vendors. Since 2010, it has been commercially available as a designer drug alternative to MDMA.
However, it’s crucial to note that there is limited data on the pharmacological properties, metabolism, and toxicity of 5-APB in humans, coupled with its relatively brief history of human usage. To ensure safety, it is highly advisable to exercise caution, conduct thorough independent research, and implement appropriate harm-reduction practices when considering the use of this substance.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 286834-81-9 |
|---|---|
| PubChem CID | 9837232 |
| ChemSpider | 8012953 |
| UNII | 2M3825704H |
| CompTox Dashboard (EPA) | DTXSID101010106 |
| Chemical and physical data | |
| Formula | C11H13NO |
| Molar mass | 175.231 g·mol−1 |
History and culture
The synthesis of 5-APB was initially documented by a team led by medicinal chemist and psychedelic researcher David E. Nichols at Purdue University. Their research focused on understanding how the MDA dioxole ring structure interacts with serotonergic neurons. Additionally, it aimed to discover an alternative to MDMA, which was gaining attention for its potential therapeutic uses but was also associated with concerns about neurotoxicity.
Although the compound was known, documented human usage did not surface until 2010, when it became available in the research chemical market. It gained notable prominence in the UK’s “legal highs” market, often sold under “Benzofury.”
Subsequently, on June 10, 2013, 5-APB and several analogues were classified as Temporary Class Drugs in the UK following a recommendation by the ACMD. Then, on November 28, 2013, the ACMD proposed that 5-APB and related benzofurans should be designated as Class B, Schedule 1 substances. Finally, on March 5, 2014, the UK Home Office announced that 6-APB, along with all other benzofuran entactogens and many structurally related drugs, would be classified as class B drugs, with the enforcement date set for June 10, 2014.
Chemistry
5-(2-aminopropyl)benzofuran, also recognized as 5-APB, belongs to the benzofuran and phenethylamine family, characterized by its ethylamine chain and a furan ring bonded to the central benzene ring. It can also be categorized as an amphetamine derivative due to the alpha methylation of the ethylamine chain. Amphetamine-class molecules typically feature a phenethylamine core comprising a phenyl ring connected to an amino (NH2) group via an ethyl chain, with an additional methyl substitution at Rα. Notably, in 5-APB, the oxygen in the furan ring is situated at the 5 position, a placement often associated with more stimulating effects, as opposed to the 6 position, which is commonly linked to more psychedelic effects. 5-APB is frequently encountered in the form of succinate and hydrochloride salt. Notably, the hydrochloride salt variant is approximately 10% more potent by mass, necessitating dosage adjustments accordingly.
Pharmacology
5-APB operates as a triple reuptake inhibitor, affecting norepinephrine, dopamine, and serotonin reuptake. Additionally, it acts as an agonist for the 5-HT2A and 5-HT2B receptors. Some hypotheses also suggest that 5-APB may function as a releasing agent for these neurotransmitters.
It effectively elevates serotonin, norepinephrine, and dopamine neurotransmitters within the brain. It achieves this by binding to and partially obstructing the transporter proteins responsible for removing these monoamines from the synaptic cleft. Consequently, dopamine and norepinephrine can accumulate in the brain, manifesting stimulating and euphoric effects.
Subjective effects
Please be aware that the effects mentioned below are derived from the Subjective Effect Index (SEI), which relies on open research literature and anecdotal user reports, including input from contributors to PsychonautWiki. Therefore, a certain level of scepticism is advisable.
Furthermore, it’s important to note that these effects may not manifest predictably or consistently, with higher doses more likely to produce the full range of products. Additionally, elevated amounts can increase the likelihood of adverse effects, including addiction, severe injury, or even fatality ☠.
Physical:
- Stimulation
- Irregular heartbeat
- Elevated heart rate
- Muscle contractions
- Increased sweating
- Dehydration
- Appetite suppression
- Nausea
- Temporary erectile dysfunction
After:
Following the peak of a stimulant experience, the subsequent results are often adverse and uncomfortable. This phase, called the “comedown,” occurs due to neurotransmitter depletion. Typical after products encompass:
- Anxiety
- Cognitive fatigue
- Depression
- Irritability
- Suppression of motivation
- Slowed thought processes
- Prolonged wakefulness
Cognitive:
- Enhancement of empathy, affection, and sociability
- Cognitive euphoria
- Compulsive redosing
- Heightened appreciation of music
- Improved focus
- Accelerated thought processes
- Enhanced analysis
- Better memory
- Increased motivation
- Sustained wakefulness
Toxicity
Disclaimer: The information provided below is based on anecdotal user reports and contributors’ insights to PsychonautWiki. It should be approached with a critical mindset.
Toxicity and Long-Term Health Effects:
The toxicity and long-term health consequences of recreational 5-APB use have not been extensively studied scientifically, and the exact toxic dosage remains unknown. This limited knowledge is primarily due to the scarce history of human usage of 5-APB. While anecdotal evidence from individuals in the community suggests that trying this substance at low to moderate doses on an infrequent basis may not lead to discernible adverse health effects, it is crucial to recognize that complete safety cannot be guaranteed.
5-APB’s pronounced affinity for the 5-HT2B receptor raises concerns about potential cardiotoxicity with prolonged use, similar to other 5-HT2B agonists like fenfluramine and MDMA. To minimize risks, it is strongly recommended that harm reduction practices be followed when using this substance.
Tolerance and Addiction Potential:
Chronic use of 5-APB can be considered moderately addictive, carrying a high potential for abuse and the possibility of inducing psychological dependence in specific individuals. Those who develop addiction may experience cravings and withdrawal symptoms upon discontinuing use.
Over time, tolerance to many of 5-APB’s effects can develop with frequent and repeated consumption. This results in users needing larger doses to achieve the same impact. Subsequently, it takes approximately 3 to 7 days for tolerance to reduce by half and 1 to 2 weeks to return to baseline, provided no further consumption exists. Importantly, 5-APB exhibits cross-tolerance with all dopaminergic stimulants, meaning that using 5-APB may diminish the effects of other stimulants.
Psychosis:
The misuse of substances within the amphetamine chemical class at high dosages over extended periods may lead to stimulant psychosis, characterized by symptoms like paranoia, hallucinations, or delusions. Research indicates that approximately 5-15% of users do not fully recover from this condition. However, antipsychotic medications have proven effective in resolving symptoms in some cases. It’s crucial to note that psychosis rarely arises from therapeutic use.
Dangerous Interactions:
It is essential to exercise caution when combining 5-APB with other substances, as many psychoactive compounds, which may be safe on their own, can become hazardous or life-threatening when mixed. While the list below provides some known dangerous interactions, it may not cover all possibilities. Independent research should always be conducted to ensure the safety of combining two or more substances:
- 25x-NBOMe & 25x-NBOH
- Alcohol
- DXM
- MDMA
- MXE
- Dissociatives
- Other stimulants like cocaine
- Tramadol
- MAOIs
- Serotonin Syndrome Risk: Combining 5-APB with certain substances can result in dangerously elevated serotonin levels, leading to serotonin syndrome, which requires immediate medical attention and can be fatal if untreated. Substances that pose a risk include MAOIs, serotonin releasers (such as MDMA), SSRIs, SNRIs, and 5-HTP.
Legal status
Brazil: The possession, production, and sale of 5-APB are illegal in Brazil, as it is included in Portaria SVS/MS nº 344.
Germany: 5-APB falls under Anlage II BtMG (Narcotics Act, Schedule II) in Germany as of July 17, 2013.[12] Engaging in manufacturing, possession, import, export, purchase, sale, procurement, or dispensation is unlawful without proper licensing.
Japan: Effective September 16th, 2015, 5-APB is a controlled substance in Japan.
Switzerland: 5-APB is specifically designated as a controlled substance listed under Verzeichnis E in Switzerland.
United Kingdom: 5-APB is classified as a Class B drug in the United Kingdom.
United States: If intended for human consumption, 5-APB could be considered an analogue of MDA and, therefore, subject to coverage under the Federal Analogue Act in the United States.
FAQ
1. What is 5-APB?
5-APB, also known as 5-(2-aminopropyl)benzofuran, is a synthetic compound in the benzofuran class. It shares structural similarities with other entactogenic substances and can produce effects reminiscent of MDMA.
2. What are the effects of 5-APB?
The effects of 5-APB can vary, but they often include stimulation, increased sociability, enhanced empathy, cognitive euphoria, and heightened appreciation of music. Users may also experience physical effects such as increased heart rate and muscle contractions.
4. Is 5-APB legal?
The legal status of 5-APB varies by country. It is illegal in many places, including the United States, the United Kingdom, and several European countries. Always check your local laws and regulations before considering its use.
5. What are the risks associated with 5-APB use?
While the long-term health effects and toxicity of 5-APB are not well-studied, there are potential risks, including addiction, adverse psychological effects during the “comedown,” and dangerous interactions with other substances.
6. Is 5-APB safe to use?
Due to the limited research on 5-APB and the potential for adverse effects, its safety cannot be guaranteed. Users are strongly encouraged to practice harm reduction, start with low doses, and avoid frequent or high-dose use.
7. Can 5-APB be tested for or detected in drug screenings?
5-APB is not typically included in standard drug screenings. However, specialized tests may be able to detect its presence. Awareness of the specific substances being tested for in any drug screening is essential.
8. What is the recommended dosage of 5-APB?
Dosage recommendations can vary depending on individual tolerance and sensitivity. It is crucial to start with a low dose, around 50-100 milligrams, and proceed cautiously. Avoid excessive or frequent dosing.
9. Can 5-APB be used for therapeutic purposes?
There is limited research on the therapeutic potential of 5-APB. It is not approved for medical use in most countries and is primarily encountered as a recreational substance.
10. What should I do in case of an adverse reaction or overdose?
If you or someone you know experiences adverse effects or a suspected overdose, seek medical attention immediately. Be honest with healthcare professionals about substance use to ensure proper care.
References
- Monte, A. P., Marona-Lewicka, D., Cozzi, N. V., Nichols, D. E. (November 1993). “Synthesis and pharmacological examination of benzofuran, indan, and tetralin analogs of 3,4-(methylenedioxy)amphetamine”. This research paper explores the synthesis and pharmacological properties of compounds related to 3,4-(methylenedioxy)amphetamine, including benzofuran analogs like 5-APB.
- EMCDDA–Europol 2010 Annual Report on the implementation of Council Decision 2005/387/JHA. This report provides insights into the implementation of regulations concerning psychoactive substances, including those related to 5-APB.
- Advisory Council on the Misuse of Drugs, Jeremy Browne (4 June 2013). “Temporary class drug order on benzofury and NBOMe compounds – letter from ACMD”. This letter discusses the temporary classification of benzofury compounds, shedding light on regulatory actions related to 5-APB.
- The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014. This legal document outlines amendments to the Misuse of Drugs Act, including changes that affect substances like 5-APB.
- Dawson, P., Opacka-Juffry, J., Moffatt, J. D., Daniju, Y., Dutta, N., Ramsey, J., Davidson, C. (3 January 2014). “The effects of benzofury (5-APB) on the dopamine transporter and 5-HT2-dependent vasoconstriction in the rat”. This scientific study delves into the effects of 5-APB on neurotransmitter systems and vascular function in rats.
- Iversen, L., Gibbons, S., Treble, R., Setola, V., Huang, X.-P., Roth, B. L. (30 January 2013). “Neurochemical profiles of some novel psychoactive substances”. This research examines the neurochemical profiles of various novel psychoactive substances, shedding light on their potential effects.
- Talaie, H.; Panahandeh, R.; Fayaznouri, M. R.; Asadi, Z.; Abdollahi, M. (2009). “Dose-independent occurrence of seizure with tramadol”. This study explores the occurrence of seizures associated with tramadol, providing insights into the potential risks of certain substances.
- Gillman, P. K. (2005). “Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity”. This article discusses the interactions between monoamine oxidase inhibitors, opioid analgesics, and serotonin toxicity, which can have implications for substances like 5-APB.
- Gillman, P. K. (2005). “Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity”. Similar to the previous entry, this article provides information on interactions between substances that affect serotonin levels and their potential toxic effects.
- Link to Brazilian Regulatory Document. This document from Brazil’s regulatory authority discusses regulations related to substances like 5-APB.
- “Anlage II BtMG” (in German). This German legal reference classifies controlled substances, including 5-APB, under the Narcotics Act, Schedule II.
- “Siebenundzwanzigste Verordnung zur Änderung betäubungsmittelrechtlicher Vorschriften” (in German). This document outlines changes in narcotics-related regulations, which can impact the legal status of substances like 5-APB.
- “§ 29 BtMG” (in German). This section of the German Narcotics Act discusses penalties and legal consequences for activities related to controlled substances like 5-APB.
- “危険ドラッグの成分4物質を新たに指定薬物に指定” (in Japanese). This Japanese government document designates certain substances, including 5-APB, as controlled drugs.
- “Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien” (in German). This Swiss legal document lists controlled substances, including 5-APB, under specific categories.
