The online market for designer drugs and research chemicals has increased in recent years, and one such substance that has garnered attention is Diphenidine. While this compound has gained popularity among researchers and experimenters, the sellers in this niche market deserve scrutiny.
First and foremost, potential buyers of Diphenidine should exercise caution when seeking out online vendors. The availability of this research chemical is widespread, but not all sellers can be trusted. It is essential to do thorough research and choose a reputable vendor with a track record of providing high-quality products and transparent information about their offerings.
Furthermore, the legality of Diphenidine can vary by country and region. Sellers should clarify these legal restrictions to potential buyers to ensure they are not inadvertently breaking the law. However, some unscrupulous vendors may overlook or downplay these legal concerns, putting their customers at risk.
Quality control is another critical issue. Since Diphenidine is a research chemical, its purity and consistency are paramount for scientific experiments. Reliable sellers should provide detailed information about the chemical’s purity, synthesis methods, and storage recommendations. Unfortunately, not all vendors adhere to these standards, potentially compromising the integrity of research projects.
The ethical implications of selling research chemicals like Diphenidine should not be ignored. Some vendors may prioritize profits over safety, leading to irresponsible marketing and distribution practices. This can encourage recreational use and misuse, which can have serious health consequences.
- 1 Summary
- 2 History and culture
- 3 Chemistry
- 4 Pharmacology
- 5 Subjective effects
- 6 Toxicity
- 7 Legal status
- 8 FAQ
- 8.1 1. What is Diphenidine?
- 8.2 2. How is Diphenidine used?
- 8.3 3. What are the effects of Diphenidine?
- 8.4 4. Is Diphenidine legal?
- 8.5 5. Are there any health risks associated with Diphenidine use?
- 8.6 6. Can Diphenidine cause addiction?
- 8.7 7. How does Diphenidine compare to other dissociatives?
- 8.8 8. What safety precautions should I take when using Diphenidine?
- 8.9 9. Can Diphenidine cause dangerous interactions with other drugs?
- 8.10 10. Where can I legally obtain Diphenidine?
- 9 References
Summary
Diphenidine, also known as DPD, DND, 1,2-DEP, and DPH, belongs to the lesser-known category of novel dissociative compounds within the diarylethylamine class. Its structural resemblance is notable, sharing similarities with methoxphenidine (MXP) and phenetidine. It operates as an NMDA receptor antagonist functionally, making it relevant in psychopharmacology.
Interestingly, the original synthesis of diphenidine dates back to 1924, though it didn’t initially receive much attention for further development. However, in the wake of the 2013 UK arylcyclohexylamine ban, piperidine, along with the closely related compound methoxphenidine, emerged on the grey market. In 2014, two documented instances of piperidine were distributed alongside synthetic cannabinoids in Japanese herbal incense blends, one of which tragically led to a fatal overdose.
Subjectively, piperidine use can elicit various effects, encompassing stimulation, motor control impairment, pain relief, internal hallucinations, conceptual thinking, euphoria, and dissociation. Dissociation, in particular, characterizes this compound’s mental state, marked by perceptual distortions and feelings of detachment from the environment and oneself. Reports have even described high doses of diphenidine resulting in “bizarre somatosensory phenomena and transient anterograde amnesia.” These effects draw parallels with arylcyclohexylamines such as ketamine and phencyclidine (PCP), as well as dextromethorphan (DXM).
Unfortunately, there is a notable scarcity of data concerning piperidine’s pharmacological properties, metabolism, and human toxicity profile. With its limited history of human usage and the potential for unique toxicity risks, some caution is warranted. Additionally, its moderate to high abuse potential underscores the importance of harm reduction practices for those considering its use. As with any novel compound, further research is necessary to comprehensively understand its properties and effects.
| Identifiers | |
|---|---|
| show IUPAC name | |
| CAS Number | 36794-52-2 |
| PubChem CID | 206666 |
| ChemSpider | 179031 |
| UNII | H8Q4VPL82Y |
| CompTox Dashboard (EPA) | DTXSID50724547 |
| Chemical and physical data | |
| Formula | C19H23N |
| Molar mass | 265.400 g·mol−1 |
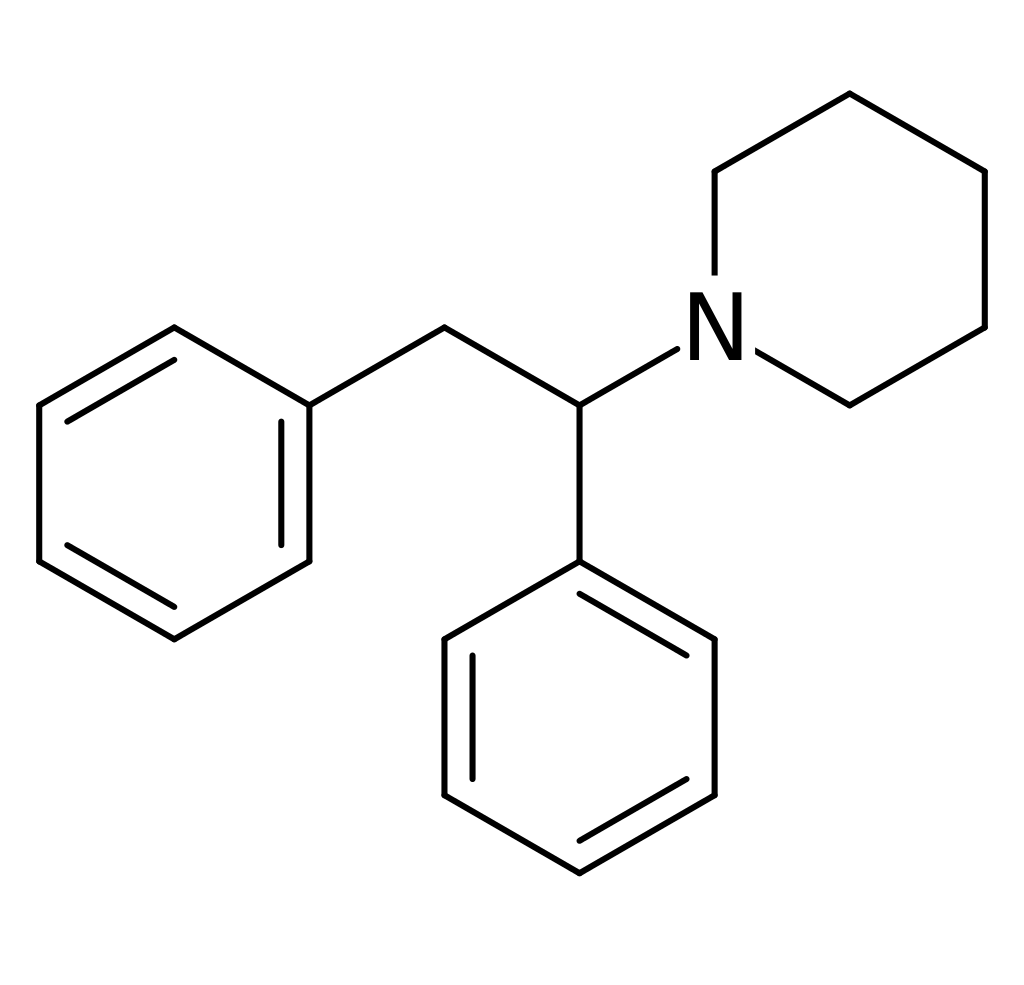
History and culture
The piperidine synthesis dates back to its initial report in 1924, employing a nitrile displacement reaction that resembles the one eventually used to discover phencyclidine in 1956. Notably, this synthesis method laid the foundation for further research into related compounds.
Following the UK’s ban on arylcyclohexylamines in 2013, the landscape of psychoactive substances shifted. Diphenidine and its closely related compound methoxphenidine swiftly emerged on the grey market as accessible options for consumers.
However, this newfound availability also brought about certain risks. In 2014, two noteworthy cases highlighted the potential dangers associated with diphenidine. The first involved piperidine being incorporated into synthetic cannabinoids within Japanese herbal incense blends. Specifically, one blend was discovered to contain diphenidine and 5-fluoro-AB-PINACA at concentrations of 289 mg/g and 55.5 mg/g, respectively. In another tragic incident, a product containing AB-CHMINACA, 5F-AMB, and piperidine was implicated in a fatal overdose, underscoring the need for caution when dealing with such substances.
Chemistry
Diphenidine falls within the diaryl ethylamine class of molecules. Its fundamental structure features a phenethylamine with a substituted Rα in the phenyl ring. Additionally, at the terminals of the piperidine ring, an amino group is linked via the phenethylamine chain. Consequently, diphenidine is categorized under the piperidine dissociative class of compounds. Notably, it shares a structural resemblance with MXP, with the key distinction being the absence of a 2-methoxy substitution on one of its phenyl rings.
Pharmacology
Diphenidine functions as an NMDA receptor antagonist,[5][6] primarily affecting the transmission of electrical signals among neurons in the brain and spinal column. In essence, these receptors must remain open for signals to traverse. Dissociatives, like diphenidine, obstruct NMDA receptors, leading to a disconnection between neurons, resulting in the characteristic loss of sensation, impaired movement, and the often-described “hole” phenomenon.
While certain vendors have asserted that piperidine acts as a dopamine reuptake inhibitor and a serotonin reuptake inhibitor, along with µ-opioid affinity, and produces typical dissociative effects, no formal screening for its affinity at the dopamine transporter has been conducted as of yet. Nevertheless, if these claims prove accurate, they could elucidate the compound’s euphoric and sometimes stimulating properties.
Diphenidine and related diarylethylamines have been explored in vitro as potential remedies for neurotoxic injuries. It is conceivable that diphenidine might serve as a more potent NMDA receptor antagonist for neurogenesis, neurological recovery, and neuroprotection compared to other commonly employed NMDA receptor antagonistic dissociatives, including ketamine, dextromethorphan, PCP analogs, Iboga, and methoxetamine.
Subjective effects
The effects of diphenidine encompass a broad spectrum of physical, visual, and cognitive experiences, varying depending on dosage and individual factors. It’s important to note that the following effects are based on anecdotal user reports and may not occur predictably or reliably, with higher doses carrying an increased risk of adverse effects and potential harm.
Physical:
- Stimulation
- Perception of bodily lightness: A sensation of weightlessness that encourages physical activity.
- Changes in felt bodily form
- Spatial disorientation
- Spontaneous bodily sensations: A tingling sensation, typically felt in the hands, feet, and head.
- Tactile suppression: Partial to complete numbness of the sense of touch, contributing to its anesthetic properties.
- Physical autonomy
- Increased salivation
- Increased heart rate
- Motor control loss: Impaired gross and fine motor control, balance, and coordination, especially at higher doses.
- Gait alteration
- Euphoria: Ranging from mild pleasure to profound bliss.
- Dizziness (uncommon)
- Nausea: Occurs at high doses but may not be as unpleasant due to detachment from physical senses.
- Orgasm suppression and sometimes enhancement, though not reliable.
Visual:
- Visual suppression
- Visual acuity suppression
- Double vision: Particularly prominent at moderate to high doses, hindering reading.
- Pattern recognition suppression: Inability to recognize and interpret visual data.
- Frame rate suppression
- Distortions
- Perspective distortions
- Environmental cubism
- Environmental orbs
- Scenery slicing
- Geometry: Dark, simplistic, and multicolored visual geometry, distinct from other dissociatives.
- Hallucinatory states, including internal hallucination.
Cognitive:
- Anxiety suppression
- Disinhibition
- Depersonalization
- Derealization
- Creativity enhancement
- Dream potentiation
- Memory suppression
- Ego death
- Ego inflation
- Thought deceleration
- Increased music appreciation
- Analysis suppression
- Time distortion
- Cognitive euphoria
- Introspection
- Déjà vu
- Conceptual thinking
- Compulsive redosing
- Amnesia
- Increased libido (reported at low to mid dosages)
- Disconnective effects, including tactile, consciousness, and visual disconnection.
Auditory:
- Auditory enhancement
- Auditory suppression
- Auditory distortion
- Auditory hallucination
It’s crucial to approach diphenidine use cautiously, considering the potential risks associated with varying dosages and individual reactions. Harm reduction practices are strongly recommended to minimize potential adverse effects and ensure safety during consumption.
Toxicity
The toxicity and potential long-term health effects of recreational diphenidine use have not been extensively studied within the scientific community, and the precise toxic dosage remains unknown. This limited research can be attributed to the scarce historical usage of diphenidine among humans.
Anecdotal reports have suggested that diphenidine use may elevate the risk of experiencing mania and psychosis, a phenomenon commonly associated with various dissociatives, particularly those that induce stimulation, such as PCP. Additionally, regular use of diphenidine has been linked to increased blood pressure and a rapid heart rate.
It is strongly recommended that individuals prioritize harm reduction practices when using this substance to mitigate potential risks and ensure their safety.
Dependence and Abuse Potential:
Similar to other NMDA receptor antagonists, chronic use of diphenidine may be associated with moderate addictive potential and a heightened risk of abuse. Certain users may develop psychological dependence, potentially leading to cravings and withdrawal effects upon discontinuation.
Tolerance Development:
Extended and repetitive use of diphenidine results in the development of tolerance, requiring users to administer progressively larger doses to achieve the desired effects. Following cessation, it typically takes 3 to 7 days for the tolerance to decrease by half and 1 to 2 weeks to return to baseline levels, provided there is no further consumption. It’s important to note that diphenidine exhibits cross-tolerance with all dissociatives, diminishing their effects after diphenidine consumption.
Dangerous Interactions:
Combining psychoactive substances, even those considered safe when used individually, can pose substantial dangers and potentially become life-threatening. Some known dangerous interactions with diphenidine include:
- Stimulants: Combining stimulants and dissociatives increases the risk of adverse psychological reactions such as anxiety, mania, delusions, and psychosis.
- Depressants: Since both depress the respiratory system, this combination elevates the risk of sudden unconsciousness, vomiting, and suffocation due to impaired breathing. In cases of nausea or vomiting, users should attempt to fall asleep in recovery or have someone assist them.
Legal status
- Canada: As of March 2016, MT-45 and its analogs, including Diphenidine, have been classified as Schedule I controlled substances. Possession without legal authorization may lead to a maximum sentence of 7 years imprisonment. Only individuals associated with law enforcement agencies, those holding exemption permits, or authorized institutions under the Minister’s approval can possess this substance.
- Switzerland: Diphenidine is explicitly categorized as a controlled substance listed under Verzeichnis E.
- Turkey: Diphenidine falls under the classification of a drug and is strictly prohibited for possession, production, supply, or import.
- United Kingdom: The production, supply, or import of this substance is illegal in the United Kingdom following the implementation of the Psychoactive Substance Act on May 26, 2016.
FAQ
1. What is Diphenidine?
- Diphenidine is a novel dissociative substance in the diaryl ethylamine class, known for its dissociative and hallucinogenic effects. It acts as an NMDA receptor antagonist.
2. How is Diphenidine used?
- Diphenidine is typically consumed orally but can also be vaporized or smoked. Users often ingest it in powder form or as part of herbal blends.
3. What are the effects of Diphenidine?
- Diphenidine’s effects may include stimulation, pain relief, euphoria, dissociation, and perceptual distortions. It can also lead to altered sensory perceptions and hallucinatory experiences.
4. Is Diphenidine legal?
- The legal status of Diphenidine varies by country. In some nations, it is classified as a controlled substance and is illegal to possess, produce, or distribute. In others, it exists in a legal gray area.
5. Are there any health risks associated with Diphenidine use?
- The long-term health effects of Diphenidine use are not well-studied. Some users have reported risks of mania, psychosis, increased blood pressure, and rapid heart rate. Like many dissociatives, it has moderate abuse potential.
6. Can Diphenidine cause addiction?
- Yes, chronic use of Diphenidine can lead to psychological dependence, with users experiencing cravings and withdrawal effects upon discontinuation.
7. How does Diphenidine compare to other dissociatives?
- Diphenidine is often compared to other dissociatives like ketamine, PCP, and DXM. It may have a more euphoric and clear-headed headspace at lower doses but can become confusing and dysphoric at higher doses.
8. What safety precautions should I take when using Diphenidine?
- It is strongly recommended to practice harm reduction when using Diphenidine. This includes starting with low doses, having a sober trip sitter present, and avoiding combining it with other substances.
9. Can Diphenidine cause dangerous interactions with other drugs?
- Yes, Diphenidine can have dangerous interactions with stimulants and depressants, potentially leading to adverse psychological reactions, respiratory depression, and an increased risk of unconsciousness and choking.
10. Where can I legally obtain Diphenidine?
- Diphenidine is often available through online research chemical vendors. However, the legality of its possession and distribution can vary significantly depending on your country’s regulations.
Please note that Diphenidine use carries potential risks, and responsible usage and awareness of local laws are essential. It’s advisable to prioritize your safety and well-being when considering its consumption.
References
- Wallach, J., Kang, H., Colestock, T., Morris, H., Bortolotto, Z. A., Collingridge, G. L., Lodge, D., Halberstadt, A. L., Brandt, S. D., Adejare, A. (17 June 2016). Lee, J., ed. “Pharmacological Investigations of the Dissociative ‘Legal Highs’ Diphenidine, Methoxphenidine and Analogues”. PLOS ONE. 11 (6): e0157021. doi:10.1371/journal.pone.0157021. ISSN 1932-6203.
- Morris, H., Wallach, J. (July 2014). “From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs: PCP to MXE.” Drug Testing and Analysis. 6 (7–8): 614–632. doi:10.1002/dta.1620. ISSN 1942-7603.
- Wurita, A., Hasegawa, K., Minakata, K., Watanabe, K., Suzuki, O. (1 August 2014). “A large amount of new designer drug diphenidine coexisting with a synthetic cannabinoid 5-fluoro-AB-PINACA found in a dubious herbal product”. Forensic Toxicology. 32 (2): 331–337. doi:10.1007/s11419-014-0240-y. ISSN 1860-8973.
- Hasegawa, K., Wurita, A., Minakata, K., Gonmori, K., Nozawa, H., Yamagishi, I., Watanabe, K., Suzuki, O. (1 January 2015). “Postmortem distribution of AB-CHMINACA, 5-fluoro-AMB, and diphenidine in body fluids and solid tissues in a fatal poisoning case: usefulness of adipose tissue for detection of the drugs in unchanged forms”. Forensic Toxicology. 33 (1): 45–53. doi:10.1007/s11419-014-0245-6. ISSN 1860-8973.
- Berger, M. L., Schweifer, A., Rebernik, P., Hammerschmidt, F. (1 May 2009). “NMDA receptor affinities of 1,2-diphenylethylamine and 1-(1,2-diphenylethyl)piperidine enantiomers and related compounds”. Bioorganic & Medicinal Chemistry. 17 (9): 3456–3462. doi:10.1016/j.bmc.2009.03.025. ISSN 0968-0896.
- Wallach, J., Kavanagh, P. V., McLaughlin, G., Morris, N., Power, J. D., Elliott, S. P., Mercier, M. S., Lodge, D., Morris, H., Dempster, N. M., Brandt, S. D. (May 2015). “Preparation and characterization of the ‘research chemical’ diphenidine, its pyrrolidine analog, and their 2,2-diphenyl ethyl isomers: Characterization of phenylethylamine”. Drug Testing and Analysis. 7 (5): 358–367. doi:10.1002/dta.1689. ISSN 1942-7603.
- Gray, N. M., Cheng, B. K., 1,2-diarylethylamines for treatment of neurotoxic injury.
- Government of Canada, P. W. and G. S. C. (2016), Canada Gazette – Regulations Amending the Food and Drug Regulations (Parts G and J — Lefetamine, AH-7921, MT-45 and W-18).
- “Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien” (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- Turkish Official Gazette, January 12, 2017. (PDF)
