When purchasing research chemicals, particularly Methoxphenidine, it’s essential to exercise caution and conduct thorough research on potential sellers. The online market for designer drugs and research chemicals has expanded considerably in recent years, leading to an influx of vendors claiming to offer quality products. However, a critical review of these sellers reveals a landscape fraught with risks and uncertainties.
Firstly, the issue of seller credibility looms large in the realm of research chemicals. Many online vendors claim to have Methoxphenidine for sale, but the legitimacy of their products often remains questionable. Buyers must navigate a labyrinth of websites, each promising the highest quality substances, making it challenging to identify trustworthy sources.
Moreover, the nature of Methoxphenidine as a research chemical adds another layer of complexity. Since it falls outside the purview of regulated substances, oversight, and quality control are minimal. This lack of regulation makes it difficult for buyers to ascertain the purity and safety of the product they purchase.
Another concerning aspect is the inconsistent pricing across different sellers. Prices for Methoxphenidine can vary significantly, leading to suspicions about the quality and authenticity of cheaper options. Buyers must be cautious not to compromise their research efforts by choosing the most affordable alternatives.
Furthermore, some vendors may not prioritize customer safety or adhere to ethical practices. Reports of unscrupulous sellers misrepresenting products, providing inadequate labeling, or failing to comply with shipping regulations have surfaced. Such practices can put researchers at risk and hinder the integrity of their work.
Contents
- 1 Summary
- 2 History and culture
- 3 Chemistry
- 4 Pharmacology
- 5 Subjective effects
- 6 Toxicity
- 7 Legal status
- 8 FAQ
- 8.1 1. What is Methoxphenidine (MXP)?
- 8.2 2. What are the effects of Methoxphenidine?
- 8.3 3. How is Methoxphenidine used?
- 8.4 4. What are the risks associated with Methoxphenidine use?
- 8.5 5. Is Methoxphenidine legal?
- 8.6 6. Can Methoxphenidine be addictive?
- 8.7 7. Are there dangerous interactions with Methoxphenidine?
- 8.8 8. What harm reduction practices should I follow when using Methoxphenidine?
- 8.9 9. Is Methoxphenidine used for any medical purposes?
- 9 References
Summary
Methoxphenidine, also recognized as 2-MXP or MXP falls within the lesser-known novel dissociative compounds in the diarylethylamine class. As an NMDA antagonist, its subjective effects resemble those induced by substances like ketamine and phencyclidine (PCP). Structurally, it shares a connection with diphenidine and phenetidine.
Research has explored Methoxphenidine alongside other diarylethylamines as a potential treatment for neurotoxic injuries. Reports of its recreational use among humans began surfacing shortly after the 2013 U.K. arylcyclohexylamine ban. During this time, Methoxphenidine and diphenidine became available in powder and tablet forms through online chemical market research. Vendors initially marketed it as a substitute for the highly popular methoxetamine (MXE) despite little to no evidence supporting similar effects.
Subjective experiences associated with Methoxphenidine encompass depersonalization, disconnection, enhanced conceptual thinking, heightened music appreciation, and euphoria. Methoxphenidine belongs to a class of substances capable of inducing a hallucinogenic state often referred to as “dissociative anesthesia,” in which users feel detached from their physical bodies.
It is essential to acknowledge that data are scarce regarding the pharmacological properties, metabolism, and toxicity of Methoxphenidine, and its history of human use is exceedingly limited. Several cases of both fatal and non-fatal overdoses have been linked to the misuse of diarylethylamines. Furthermore, user reports have raised concerns about potential risks that may differ from, and possibly be more pronounced than, those associated with traditional dissociative substances.
Given these uncertainties, it is strongly recommended to implement harm-reduction practices when considering using Methoxphenidine. Users should exercise caution, prioritize safety, and remain vigilant about their well-being when engaging with this substance.
| Identifiers | |
|---|---|
| show IUPAC name | |
| CAS Number | 127529-46-8 |
| PubChem CID | 67833251 |
| ChemSpider | 52085156 |
| UNII | H2W7A6GZGX |
| Chemical and physical data | |
| Formula | C20H25NO |
| Molar mass | 295.426 g·mol−1 |
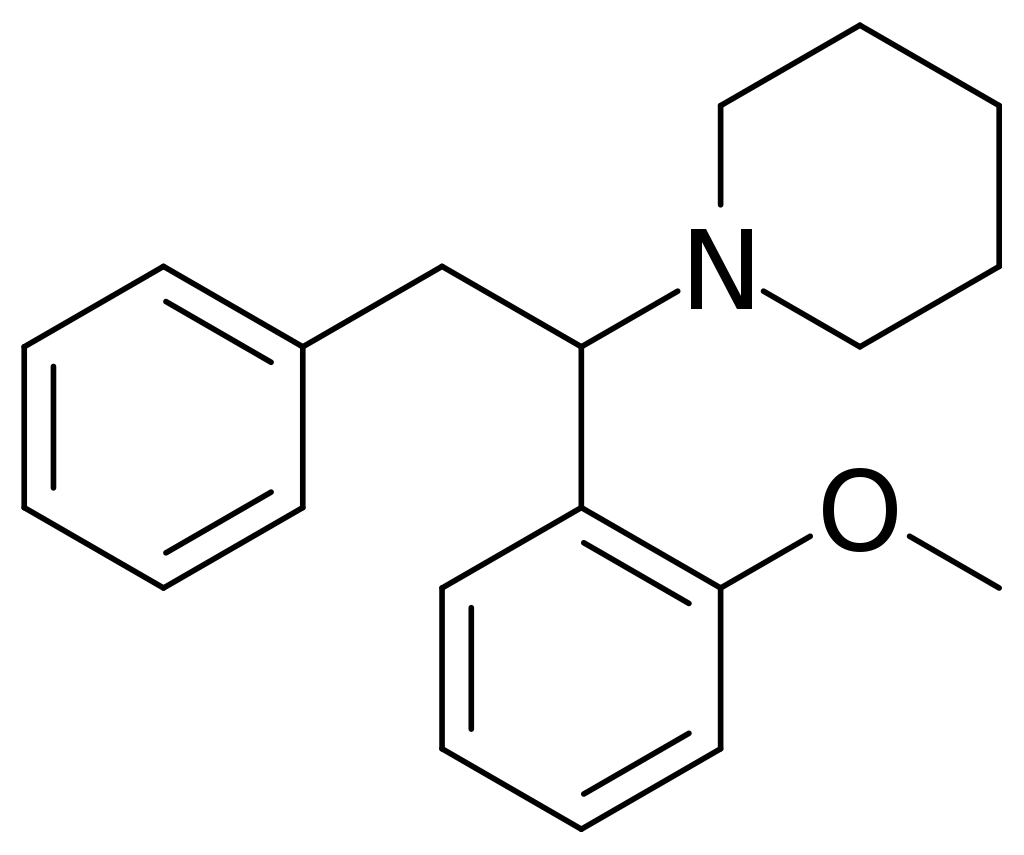
History and culture
Methoxphenidine illustrates a designer drug deliberately crafted to replicate the functional or structural characteristics of frequently used illicit substances, aiming to bypass government regulations.
Chemistry
Methoxphenidine, also known as 2-MeO-Diphenidine, is a synthetic compound classified under the diarylethylamine category. Its chemical structure comprises a substituted phenethylamine framework with an extra phenyl ring linked to Rα. The terminal amino group within the phenethylamine chain is integrated into a piperidine ring, classifying methoxphenidine within the piperidine dissociative compound class. This substance shares structural similarities with piperidine, distinguished by the presence of a methoxy group at the two-position of a phenyl group.
Pharmacology
MXP operates as an NMDA receptor antagonist,[10] affecting the N-methyl-D-aspartate (NMDA) receptors responsible for transmitting electrical signals between neurons in the brain and spinal cord. These receptors must remain open for signals to pass through. Dissociatives like MXP function by obstructing and closing these NMDA receptors. This interference in neuronal communication results in sensations of numbness and impaired mobility and ultimately resembles the distinctive experience known as the “k-hole.”
While formal research on this aspect is lacking, anecdotal reports from users suggest that MXP may also exhibit properties as a dopamine and noradrenaline reuptake inhibitor. This potential action could contribute to the physical and emotional euphoria frequently described by individuals who have used the substance.
Subjective effects
It’s essential to recognize that, similar to other diarylethylamines, methoxphenidine demonstrates a notably quicker onset and shorter half-life when vaporized or smoked. Consuming this way is considered carcinogenic when subjected to excessive heat. Some user accounts have indicated that vaporization can require as little as 20% of the typical oral dosage for an individual.
Please note that the effects outlined below are based on the Subjective Effect Index (SEI), a collection of anecdotal user reports and personal analyses from contributors at PsychonautWiki. As a result, these effects should be approached with a degree of caution.
It’s also crucial to understand that these effects may not manifest consistently or predictably. Higher doses are more likely to elicit the complete range of effects, and elevated doses carry an increased risk of adverse effects, including addiction, severe injury, or even death ☠.
Physical:
The physical effects of MXP closely resemble those of DXM rather than other commonly used dissociatives. These effects intensify progressively with dosage and include:
- Tactile Disconnection: This leads to a gradual sense of physical detachment, which can be consciously controlled to a greater extent than with other dissociatives.
- Pain Relief
- Spontaneous Physical Sensations: A pleasurable vibrating sensation known as the “MXP body high” gradually intensifies during the onset and subsides at the peak.
- Tactile Suppression: This partially or fully suppresses the sense of touch, resulting in numbness in the extremities and contributing to the substance’s anesthetic properties.
- Motor Control Loss: Users experience a decline in both gross and fine motor control, balance, and coordination. For safety, it’s recommended to be seated during the onset, especially for those not experienced with the substance.
- Euphoria generates physical euphoria, ranging from mild pleasure to profound bliss.
- Perception of Bodily Lightness: Users feel their body is weightless, encouraging physical activity at lower to moderate doses.
- Dizziness: Although infrequent, some users report dizziness under the influence of MXP.
- Physical Autonomy
- Spatial Disorientation
- Orgasm Suppression
- Gait Alteration
Visual:
Visual effects include:
- Suppression
- Visual Disconnection: This leads to experiences akin to the “K-hole,” characterized by voids, spaces, and structures.
- Visual Acuity Suppression
- Double Vision: Prevalent at moderate to high doses, making reading impossible without closing one eye.
- Pattern Recognition Suppression: Occurs at higher doses, rendering one unable to recognize and interpret visual data.
- Frame Rate Suppression
- Distortions
- Perspective Distortions
- Environmental Cubism
- Environmental Orbism
- Scenery Slicing
- Geometry
- Hallucinatory States
MXP may induce a range of high-level hallucinatory states at high doses, albeit with less consistency and predictability than many other commonly used psychedelics.
Cognitive:
The Cognitive effects of MXP are often described as remarkably clear-headed compared to other dissociatives, even at high doses. These effects include:
- Depersonalization
- Derealization
- Dream Potentiation
- Consciousness Disconnection
- Thought Acceleration
- Memory Suppression
- Ego Death
- Thought Deceleration
- Increased Music Appreciation
- Analysis Suppression
- Time Distortion: Feelings of time dilation, with more time having passed than perceived, are expected at moderate to high doses.
- Euphoria
- Conceptual Thinking
- Anxiety Suppression
- Disinhibition
- Amnesia
Auditory:
Auditory effects include:
- Suppression
- Auditory Distortions occur at moderate to high doses, presenting as a delay in sound processing. The sound may be perceived as lagging by 2-15 seconds with extremely distorted syllables at heavy doses.
- Hallucinations: Audial time dilation experienced at moderate to heavy doses can manifest as processing sound 2-5 seconds after it occurs, with a meager frame rate, echoing, and extended syllables.
After:
The afterglow refers to the effects occurring within 24 hours after the experience; many users report it as long-lasting and enjoyable as the experience itself. The afterglow includes physical sensations of euphoria, rejuvenation, relaxation, and a sense of bodily lightness. Mentally, it involves reduced or absent anxiety, increased contentment, and heightened music appreciation, typically dissipating within a day or so after the experience.
Toxicity
The toxicity and potential long-term health consequences of recreational MXP use have not undergone scientific scrutiny, and the precise toxic dosage remains unknown. This lack of knowledge stems from MXP’s limited history of human consumption.
Anecdotal accounts from individuals who have experimented with this substance suggest no apparent adverse health effects when used alone at low to moderate doses and in moderation. However, it’s important to note that nothing can be entirely guaranteed, and it’s advisable to conduct independent research to ensure the safety of combining two or more substances before consumption.
The prudent approach is to incorporate harm-reduction practices when using MXP.
Dependence and Abuse Potential:
Similar to other NMDA receptor antagonists, chronic MXP use can potentially lead to moderate addiction risk and a high likelihood of abuse, resulting in psychological dependence for specific users. When addiction develops, individuals may experience cravings and withdrawal symptoms upon discontinuation.
Tolerance to many of MXP’s effects develops with prolonged and repeated use. Consequently, users may need to administer progressively larger doses to achieve the desired effects. Tolerance reduction typically takes approximately 3 to 7 days to reach half and 1 to 2 weeks to return to baseline (assuming no further consumption). Significantly, MXP induces cross-tolerance with all dissociatives, diminishing the effects of other substances in this category.
Dangerous Interactions:
It’s essential to exercise caution when combining psychoactive substances, as interactions can unexpectedly become dangerous or life-threatening. The following list highlights some known dangerous interactions but may not cover all possibilities.
Independent research, such as consulting search engines like Google or medical databases like PubMed, is crucial to verify the safety of combining two or more substances. Some of the following interactions have been sourced from TripSit:
- Depressants (e.g., 2M2B, alcohol, barbiturates, benzodiazepines, GHB/GBL, methaqualone, opioids): This combination amplifies muscle relaxation, amnesia, sedation, and respiratory depression, potentially leading to sudden loss of consciousness, respiratory failure, and suffocation if vomiting occurs while unconscious. If nausea or vomiting arises before losing consciousness, it’s advisable to lie in the recovery position or have assistance from a friend.
- Stimulants (e.g., amphetamine, cocaine, methylphenidate, MDMA): Combining stimulants with dissociatives like MXP can intensify anxiety, manic symptoms, delusions, and disinhibition, increasing the risk of anxiety attacks, delusions, or psychotic episodes. There is also evidence suggesting heightened neurotoxicity when these substances are combined. Additionally, users often report worsened comedowns when mixing these classes of drugs.
- Dissociatives (e.g., dextromethorphan, ketamine, methoxetamine, PCP): This combination can unpredictably enhance amnesia, sedation, motor control loss, and delusions. It may also lead to sudden unconsciousness accompanied by significant respiratory depression. In cases of nausea or vomiting before losing consciousness, individuals should aim to rest in the recovery position or seek assistance from a friend.
Legal status
Canada: Since March 2016, MT-45 and its analogs, including methoxphenidine, have been classified as Schedule I controlled substances in Canada. Unauthorized possession can lead to a maximum imprisonment term of 7 years. Only law enforcement agencies, individuals with an exemption permit, or authorized institutions may legally possess the substance.
China: Methoxphenidine has been a controlled substance in China since October 2015.
Germany: Methoxphenidine is considered a controlled substance under the NpSG (New Psychoactive Substances Act) in Germany due to its derivation from 2-phenethylamine. Its production and sale are illegal, but possession and import, although unlawful, are not penalized if intended for personal consumption.
Italy: Methoxphenidine is a prohibited substance in Italy.
Sweden: Methoxphenidine is also a prohibited substance in Sweden.
Switzerland: Methoxphenidine is explicitly named as a controlled substance under Verzeichnis E in Switzerland.
Turkey: In Turkey, Methoxphenidine is classified as a drug and is illegal to possess, produce, supply, or import.
United Kingdom: The Psychoactive Substance Act, effective since May 26, 2016, makes it illegal to produce, supply, or import Methoxphenidine in the United Kingdom.
United States: As of the information available, Methoxphenidine is not explicitly scheduled in the United States. However, individuals may still face charges for its possession under certain circumstances, such as analog laws or when there is intent to sell or consume the substance. Please note that legal statuses can change, and staying updated on current regulations and laws in your jurisdiction is essential.
FAQ
1. What is Methoxphenidine (MXP)?
- Methoxphenidine, often abbreviated as MXP, is a synthetic dissociative compound that belongs to the diarylethylamine class of substances. It is structurally related to piperidine and phenetidine.
2. What are the effects of Methoxphenidine?
- Methoxphenidine produces dissociative effects similar to ketamine and phencyclidine (PCP). Users often report feelings of depersonalization, disconnective effects, conceptual thinking, increased music appreciation, and euphoria. It can induce “dissociative anesthesia,” where users feel detached from their bodies.
3. How is Methoxphenidine used?
- Methoxphenidine is typically consumed orally, although some users may choose to vaporize or smoke it. Oral consumption is the most common method, often by dissolving the substance in a liquid or encapsulating it in a gelatin capsule.
4. What are the risks associated with Methoxphenidine use?
- Methoxphenidine has limited research on its pharmacological properties and potential long-term effects. There have been reports of both fatal and non-fatal overdoses associated with its use. Some users have reported different and more pronounced risks compared to traditional dissociatives.
5. Is Methoxphenidine legal?
- The legal status of Methoxphenidine varies by country. In some nations, it is classified as a controlled or prohibited substance, making its possession, sale, or importation illegal. Researching and understanding the legal status of Methoxphenidine in your jurisdiction is crucial before obtaining or using it.
6. Can Methoxphenidine be addictive?
- Like other NMDA receptor antagonists, chronic use of Methoxphenidine may lead to psychological dependence and addiction in some users. Cravings and withdrawal effects can occur when someone stops using the substance after developing a dependence.
7. Are there dangerous interactions with Methoxphenidine?
- Yes, Methoxphenidine can interact dangerously with other substances. It’s crucial to be aware of potential interactions, especially when combined with depressants (e.g., alcohol, benzodiazepines) or stimulants (e.g., amphetamines, cocaine). These combinations can lead to adverse effects and health risks.
8. What harm reduction practices should I follow when using Methoxphenidine?
- If you choose to use Methoxphenidine, it’s essential to prioritize harm reduction. This includes starting with a low dose, using it in a safe and familiar environment, having a trusted friend present, and avoiding combining it with other substances. Additionally, always stay hydrated and be cautious of potential adverse reactions.
9. Is Methoxphenidine used for any medical purposes?
- Methoxphenidine has limited medical research and is not approved for any specific medical use. It has been studied in the context of treating neurotoxic injuries, but more research is needed to establish its safety and efficacy.
References
- Wallach, J., Kang, H., Colestock, T., Morris, H., Bortolotto, Z. A., Collingridge, G. L., Lodge, D., Halberstadt, A. L., Brandt, S. D., Adejare, A. (17 June 2016). “Pharmacological Investigations of the Dissociative’ Legal Highs’ Diphenidine, Methoxphenidine and Analogues”. PLOS ONE. 11 (6): e0157021. doi:10.1371/journal.pone.0157021. ISSN 1932-6203.
- Nancy M. Gray; Brian K. Cheng (6 April 1994). “Patent EP 0346791 – 1,2-diarylethylamines for treatment of neurotoxic injury”. G.D. Searle, LLC – via SureChEMBL.
- Michael L. Berger; Anna Schweifer; Patrick Rebernik; Friedrich Hammerschmidt (May 2009). “NMDA receptor affinities of 1,2-diphenylethylamine and 1-(1,2-diphenylethyl)piperidine enantiomers and related compounds”. Bioorganic & Medicinal Chemistry. 17 (1): 3456–3462. doi:10.1016/j.bmc.2009.03.025. PMID 19345586.
- Jason Wallach; Pierce V. Kavanagh; Gavin McLaughlin; Noreen Morris; John D. Power; Simon P. Elliott; Marion S. Mercier; David Lodge; Hamilton Morris; Nicola M. Dempster; Simon D. Brandt (May 2015). “Preparation and characterization of the ‘research chemical’ piperidine, its pyrrolidine analog, and their 2,2-diphenyl ethyl isomers”. Drug Testing and Analysis. 7 (5): 358–367. doi:10.1002/dta.1689. PMID 25044512.
- Thurkauf, Andrew; Monn, James; Mattson, Marienna V.; Jacobson, Arthur E.; Rice, Kenner C. (1989). “Structural and conformational aspects of the binding of aryl-alkyl amines to the phencyclidine binding site” (PDF). NIDA research monograph. 95: 51–56. ISSN 1046-9516. PMID 2561843.
- Goodson, L. H.; Wiegand, C. J. W.; Splitter, Janet S. (November 1946). “Analgesics. I. N-Alkylated-1,2-diphenylethylamines Prepared by the Leuckart Reaction”. Journal of the American Chemical Society. 68 (11): 2174–2175. doi:10.1021/ja01215a018. PMID 21002222.
- McLaughlin, G., Morris, N., Kavanagh, P. V., Power, J. D., O’Brien, J., Talbot, B., Elliott, S. P., Wallach, J., Hoang, K., Morris, H., Brandt, S. D. (January 2016). “Test purchase, synthesis, and characterization of 2-methoxydiphenidine (MXP) and differentiation from its meta – and para-substituted isomers: Characterization of 2-, 3-, and 4-methoxydiphenidine isomers”. Drug Testing and Analysis. 8 (1): 98–109. doi:10.1002/dta.1800. ISSN 1942-7603.
- Morris, H., Wallach, J. (August 2014). “From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs”. Drug Testing and Analysis. 6 (7–8): 614–632. doi:10.1002/dta.1620. ISSN 1942-7611.
- Van Hout, M. C., Hearne, E. (March 2015). “Word of mouse”: indigenous harm reduction and online consumerism of the synthetic compound methoxphenidine.” Journal of Psychoactive Drugs. 47 (1): 30–41. doi:10.1080/02791072.2014.974002