- 1 Explore Pyrovalerone Research Chemicals from Trusted Vendors
- 2 Convenience of Buying Pyrovalerone Online
- 3 How to Identify Trusted Pyrovalerone Vendors
- 4 Key Considerations When Buying Pyrovalerone
- 5 Why Researchers Opt for Pyrovalerone
- 6 Make the Right Choice with Pyrovalerone
- 7 Summary
- 8 Legal status
- 9 FAQ
- 9.1 1. What is Pyrovalerone?
- 9.2 2. What are some familiar brand names for Pyrovalerone?
- 9.3 3. When was Pyrovalerone developed?
- 9.4 4. In which countries is Pyrovalerone used?
- 9.5 5. What are the common side effects of Pyrovalerone?
- 9.6 6. Can Pyrovalerone lead to withdrawal symptoms?
- 9.7 7. Is there a difference between the enantiomers of Pyrovalerone?
- 9.8 8. Is Pyrovalerone available as a prescription medication?
- 9.9 9. Can Pyrovalerone be addictive?
- 9.10 10. Is Pyrovalerone legal?
- 10 References
Explore Pyrovalerone Research Chemicals from Trusted Vendors
When searching for Pyrovalerone for sale, finding authentic, high-quality options is key to ensuring reliability and consistency for research purposes. Trusted vendors offer diverse solutions, whether you’re looking to buy Pyrovalerone online in the USA or Canada, or seeking competitive pricing and secure purchasing options. Knowing how to evaluate vendors and choose the best Pyrovalerone products can streamline your process and provide peace of mind.
Convenience of Buying Pyrovalerone Online
Purchasing Pyrovalerone online has never been easier, with reputable platforms offering competitive prices and detailed product descriptions. These vendors make ordering straightforward, with options tailored for both the USA and Canada markets. By searching terms such as buy Pyrovalerone online or order Pyrovalerone USA, you’ll gain access to a wide range of products designed for research applications.
Online shops often focus on transparency, providing customers with clear labeling, exact specifications, and options like discreet shipping. For researchers, this ensures that every purchase meets authenticity and quality standards, which are critical for precise and reliable outcomes.
How to Identify Trusted Pyrovalerone Vendors
When looking for Pyrovalerone for sale or exploring Pyrovalerone buy options, it’s essential to prioritize quality over cost. Trusted Pyrovalerone vendors establish credibility by offering consistently high-purity products. These shops typically provide certificates of analysis, robust customer reviews, and secure payment methods.
Additionally, reliable vendors often specialize in research chemicals, meaning they understand the importance of appropriate handling, packaging, and delivery. Whether searching for Pyrovalerone shop options in the USA or exploring purchase Pyrovalerone boutiques in Canada, reputable sellers focus on customer satisfaction and safety.
Key Considerations When Buying Pyrovalerone
- Product Authenticity
Ensure that the Pyrovalerone products you select are accurately labeled and offered by verified vendors. Reviews and product certifications are an excellent way to confirm authenticity.
- Vendor Transparency
Look for sellers who provide detailed product descriptions, pricing breakdowns, and multiple shipping options. Terms like Pyrovalerone buy USA and Pyrovalerone online Canada often lead to platforms with verified credibility.
- Seamless Online Experience
Top vendors make the process easy by offering intuitive browsing, secure payments, and reliable customer support. Bonus incentives like bulk discounts also make ordering Pyrovalerone online more attractive.
Why Researchers Opt for Pyrovalerone
Researchers value Pyrovalerone for its distinct chemical properties and wide-ranging applications in experimental scenarios. By choosing well-established Pyrovalerone vendors focused on the research market, professionals can avoid the uncertainties associated with substandard sources. Trusting experienced sellers ensures that your materials are suitable for research while meeting regulatory standards.
Make the Right Choice with Pyrovalerone
Between local and online options, finding Pyrovalerone for sale that matches your requirements is easier when you prioritize trusted vendors. Whether you’re exploring Pyrovalerone buy Canada offers or searching for a reputable Pyrovalerone shop in the USA, the key is to align quality, price, and reliability. Take time to review vendor authenticity, product details, and shipping options to ensure every purchase is up to your expectations.
Summary
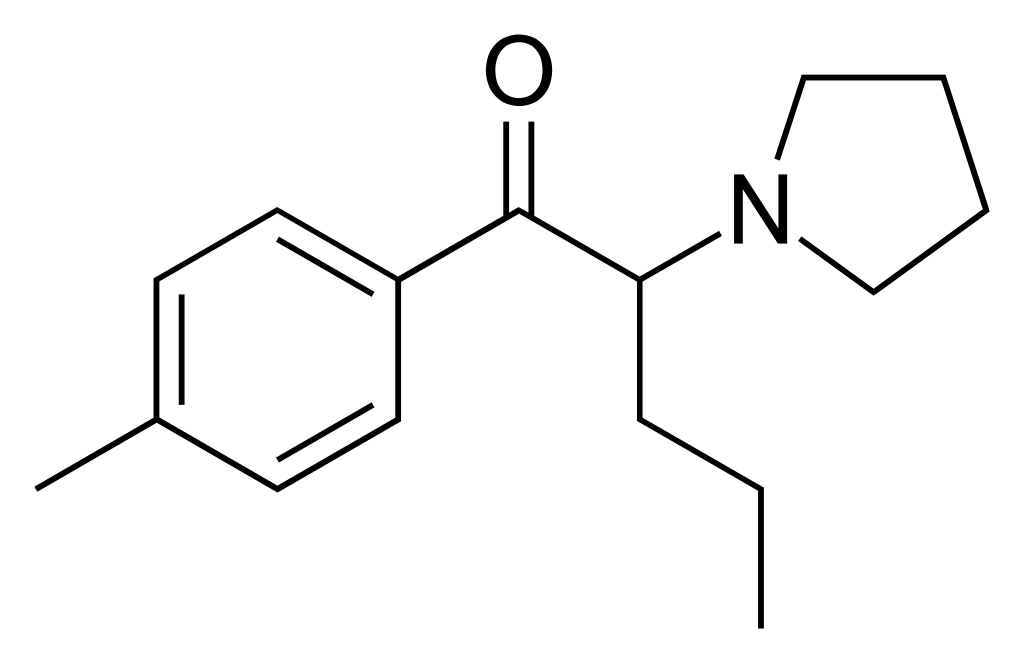
Pyrovalerone, also known as Centroton, 4-Methyl-β-keto-prolintane, Thymergix, or O-2371, is a psychoactive substance known for its stimulant properties. It achieves these effects by functioning as a norepinephrine-dopamine reuptake inhibitor (NDRI). This compound has been utilized in clinical settings for managing chronic fatigue and lethargy and as an appetite suppressant for weight loss.
Initially developed in the late 1960s, pyrovalerone found use in France and several other European countries. However, its prescription has dwindled over time due to concerns related to misuse and dependence. Pyrovalerone is similar to various other stimulants, including α-PVP, MDPV, and prolintane (Promotil, Katovit).
Some common side effects associated with pyrovalerone usage encompass reduced appetite or anorexia, heightened anxiety, disrupted sleep patterns or insomnia, and muscle tremors or trembling. Discontinuing its use after prolonged abuse can often lead to symptoms of depression.
It’s worth noting that the R-enantiomer of pyrovalerone does not exhibit any significant activity.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 3563-49-3 1147-62-2 (hydrochloride) |
|---|---|
| PubChem CID | 14373 |
| ChemSpider | 13733 |
| UNII | VOU69C02JP |
| KEGG | D05663 |
| ChEMBL | ChEMBL201960 |
| CompTox Dashboard (EPA) | DTXSID20863194 |
| ECHA InfoCard | 100.230.426 |
| Chemical and physical data | |
| Formula | C16H23NO |
| Molar mass | 245.366 g·mol−1 |
Legal status
| Legal status | |
|---|---|
| Legal status | AU: S4 (Prescription only) BR: Class B1 (Psychoactive drugs)[1] CA: Schedule IV DE: Anlage II (Authorized trade only, not prescriptible) UK: Class C US: Schedule V |
FAQ
1. What is Pyrovalerone?
Pyrovalerone is a psychoactive substance known for its stimulant effects. It operates as a norepinephrine-dopamine reuptake inhibitor (NDRI) and has been utilized in clinical settings to manage chronic fatigue, lethargy, and weight loss.
2. What are some familiar brand names for Pyrovalerone?
Pyrovalerone is marketed under various brands, including Centroton, 4-Methyl-β-keto-prolintane, Thymergix, and O-2371.
3. When was Pyrovalerone developed?
Pyrovalerone was initially developed in the late 1960s.
4. In which countries is Pyrovalerone used?
Pyrovalerone has been used in France and several other European countries. However, its usage has decreased over time due to concerns regarding misuse and dependence.
5. What are the common side effects of Pyrovalerone?
Side effects associated with Pyrovalerone use may include reduced appetite or anorexia, increased anxiety, disrupted sleep patterns or insomnia, and muscle tremors or trembling.
6. Can Pyrovalerone lead to withdrawal symptoms?
Yes, discontinuing using Pyrovalerone after prolonged abuse can often result in withdrawal symptoms, including depression.
7. Is there a difference between the enantiomers of Pyrovalerone?
Yes, the R-enantiomer of Pyrovalerone does not exhibit significant activity.
8. Is Pyrovalerone available as a prescription medication?
While Pyrovalerone was previously prescribed for specific medical conditions, its prescription and usage have become infrequent due to concerns related to abuse and dependence.
9. Can Pyrovalerone be addictive?
Yes, Pyrovalerone has the potential for abuse and dependence, which is one of the reasons for its decreased usage in clinical settings.
10. Is Pyrovalerone legal?
The legal status of Pyrovalerone can vary by country and region. To determine their legality, it is essential to check your area’s specific regulations and laws.
References
- Anvisa, the Brazilian health regulatory agency, published Collegiate Board Resolution No. 784 on March 31, 2023. This resolution contains lists of substances categorized as narcotic, psychotropic, precursor, and other substances under special control in Brazil. It was officially published in the Diário Oficial da União on April 4, 2023, and can be accessed through the official archives until August 3, 2023.
- Pyrovalerone was the subject of US Patent 3314970, indicating its history of research and development.
- In October 1971, a study titled “Evaluation of pyrovalerone in chronically fatigued volunteers” was conducted by Gardos G and Cole JO, which assessed the effects of pyrovalerone on individuals experiencing chronic fatigue.
- In November 1975, a report titled “[Abuse of pyrovalerone by drug addicts]” was published by Deniker P, Lôo H, Cuche H, and Roux JM, highlighting issues related to the misuse of pyrovalerone among drug addicts.
- In February 2006, Meltzer PC, Butler D, Deschamps JR, and Madras BK conducted research on analogues of pyrovalerone, a compound known as “1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone).” These analogues were investigated as potential monoamine uptake inhibitors.
