Beautiful Plants For Your Interior
Summary
Dextropropoxyphene, also recognized as Propoxyphene and Darvon, is a synthetic opioid belonging to the phenylpropylamine chemical group. Similar to other compounds within this category, like tapentadol and tramadol, it elicits mild euphoric, analgesic, soothing, and antitussive effects when administered, typically through oral ingestion, though occasionally intravenous or rectal administration may occur.
It is worth noting that dextropropoxyphene is reported to induce notably less euphoria when compared to other opioids.
Eli Lilly and Company initially patented dextropropoxyphene in 1955 and subsequently manufactured it.
Due to its potential for euphoria and pain relief, dextropropoxyphene is recognized as having addictive properties, although to a lesser extent than more potent opioids such as morphine or heroin. It’s crucial to be aware that dextropropoxyphene can also lead to seizures and potentially life-threatening cardiac arrhythmias at high doses, and these effects cannot be reversed using naloxone.
Currently, dextropropoxyphene is seldom encountered illicitly and may sometimes be obtained through prescription from compounding pharmacies. It is strongly advisable for individuals to thoroughly research the substance’s toxicity and implement appropriate harm reduction measures if they decide to use this compound.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 469-62-5 |
|---|---|
| PubChem CID | 10100 |
| IUPHAR/BPS | 7593 |
| DrugBank | DB00647 |
| ChemSpider | 9696 |
| UNII | S2F83W92TK |
| KEGG | D07809 |
| ChEBI | CHEBI:51173 |
| ChEMBL | ChEMBL1213351 |
| CompTox Dashboard (EPA) | DTXSID1023524 |
| ECHA InfoCard | 100.006.747 |
| Chemical and physical data | |
| Formula | C22H29NO2 |
| Molar mass | 339.479 g·mol−1 |
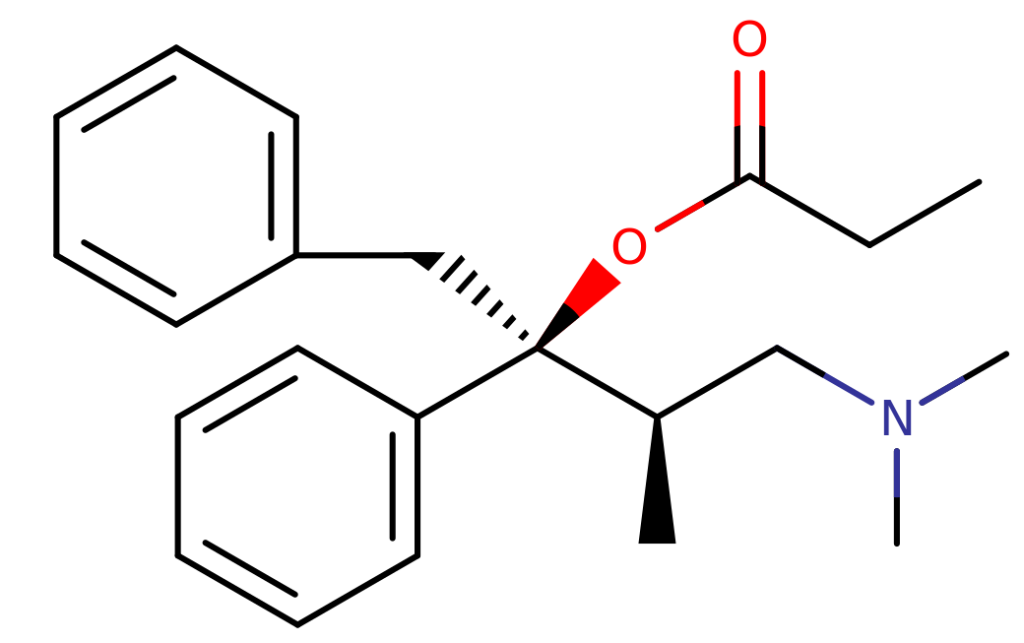
Chemistry
Dextropropoxyphene exhibits structural similarities to tapentadol. While tapentadol features an ethyl substitution on the gamma-carbon, dextropropoxyphene, on the other hand, presents both benzyl and propionyl substitutions. Dextropropoxyphene also includes a benzene ring instead of the phenol ring observed in tapentadol. The empirical formula for dextropropoxyphene is C22H29NO2, with a molar mass of 339.471 grams per mole.
Pharmacology
Opioids exert their effects by binding to and activating the μ-opioid receptor. This mechanism arises from the structural resemblance between opioids and endogenous endorphins, which naturally occur in the body and also interact with the μ-opioid receptor system. Opioids’ ability to mimic these natural endorphins is responsible for their capacity to induce euphoria, alleviate pain, and produce anxiolytic effects. Endorphins, in their natural role, play a vital part in pain reduction, sleep induction, and the generation of pleasurable sensations. They can be released in response to various stimuli, such as pain, strenuous physical activity, orgasms, or moments of heightened excitement.
Dextropropoxyphene, unlike most opioids, possesses additional properties as a weak serotonin reuptake inhibitor and a potent antagonist of nicotinic acetylcholine receptors. This compound exhibits a bioavailability of approximately 40% and undergoes metabolism primarily by the cytochrome P450 3A4 enzyme. Interestingly, its optical isomer, levopropoxyphene, lacks analgesic activity but retains antitussive effects.
Subjective effects
Disclaimer: The effects listed below are sourced from the Subjective Effect Index (SEI), a collection of anecdotal user reports and personal analyses contributed by PsychonautWiki contributors. Therefore, it is important to approach these effects with a degree of skepticism.
It is also essential to recognize that these effects may not necessarily manifest predictably or consistently, with higher doses increasing the likelihood of experiencing the full range of effects. Moreover, higher doses can raise the risk of adverse effects, including addiction, severe injury, or even fatal outcomes ☠.
Physical:
- Euphoria and Relaxation: Dextropropoxyphene often induces a general sense of euphoria, relaxation, anxiety reduction, and pain relief.
- Pain Relief: While dextropropoxyphene provides pain relief, its effectiveness in this regard is generally milder compared to opioids like morphine and fentanyl.
- Physical Comfort: This substance may produce physical euphoria, albeit less intensely than morphine or diacetylmorphine (heroin). Users might describe it as extreme physical comfort, warmth, love, and bliss.
- Itchiness
- Respiratory Depression: At low to moderate doses, dextropropoxyphene mildly to moderately slows down breathing without causing significant impairment. However, high doses or overdoses can lead to noticeable shortness of breath, abnormal breathing patterns, semi-consciousness, or unconsciousness. Severe overdoses may result in coma or death without prompt medical attention.
- Constipation
- Cough Suppression
- Difficulty Urinating
- Nausea
- Sedation
- Pupil Constriction
- Decreased Libido
- Appetite Suppression
- Dizziness: Dextropropoxyphene is more likely to cause dizziness compared to other opioids.
- Orgasm Suppression
Cognitive:
- Cognitive Euphoria: Dextropropoxyphene may induce cognitive euphoria, but it tends to be less intense than that of morphine or diacetylmorphine (heroin). Users may describe it as a powerful and overwhelming sensation of emotional bliss, contentment, and happiness.
- Anxiety Suppression
- Compulsive Redosing
- Confusion: Dextropropoxyphene may lead to confusion at a higher rate than other opioids.
- Dream Potentiation
Toxicity
Disclaimer: The effects and interactions listed below are sourced from the Subjective Effect Index (SEI), a compilation of anecdotal user reports and personal analyses contributed by PsychonautWiki contributors. Exercise caution and skepticism when considering these effects.
High Toxicity:
Dextropropoxyphene exhibits a significant level of toxicity relative to its dose. Like all opioids, long-term effects can vary, potentially leading to diminished libido, apathy, and memory loss.
Dangerous Combinations:
Dextropropoxyphene poses notable risks when combined with other substances, especially depressants like alcohol or benzodiazepines. It has a broader range of potentially dangerous interactions compared to many other opioids.
Seizure Threshold:
Dextropropoxyphene is known to lower the seizure threshold. Individuals going through benzodiazepine withdrawal should avoid using it, as it can potentially trigger seizures.
Cardiac Arrhythmias:
Dextropropoxyphene is associated with potentially fatal heart arrhythmias. Heavy dosing over consecutive days or very high doses should be avoided.
Harm Reduction Practices:
It is strongly recommended to employ harm reduction practices when using this substance.
Tolerance and Addiction Potential:
Chronic use of dextropropoxyphene can lead to moderate addiction potential, with a high likelihood of abuse and the potential for psychological dependence. Users may experience cravings and withdrawal symptoms upon abrupt cessation.
Tolerance Development:
Tolerance develops at varying rates for different effects, with some effects, like constipation, developing tolerance slowly. Users may need to increase their doses to achieve the same effects. Tolerance to dextropropoxyphene extends to other opioids, reducing their effectiveness.
Dangerous Interactions:
Combining dextropropoxyphene with certain substances can be life-threatening. Here are some known dangerous interactions:
- Alcohol: Combining alcohol with dextropropoxyphene can lead to ataxia, sedation, and potential loss of consciousness. Aspiration due to vomiting is a risk.
- Amphetamines: Stimulants can increase respiration rates, allowing higher opioid doses. However, if the stimulant wears off before the opioid, respiratory arrest may occur.
- Benzodiazepines: Combining benzodiazepines and dextropropoxyphene can result in central nervous system and respiratory depression, potentially leading to unconsciousness. Aspiration due to vomiting is a risk.
- Cocaine: Stimulants like cocaine can increase respiration rates, allowing for higher opioid doses. However, if the stimulant effects dissipate first, the opioid may overwhelm the user, causing respiratory arrest.
- DXM: Combining dextropropoxyphene with DXM is generally considered toxic, leading to central nervous system depression, breathing difficulties, heart issues, and liver toxicity. It can also reduce opioid tolerance, intensifying their combined effects.
- GHB/GBL: These substances can strongly and unpredictably potentiate the effects of dextropropoxyphene, potentially leading to rapid unconsciousness. Aspiration due to vomiting is a risk.
- Ketamine: Both substances can increase the risk of vomiting and unconsciousness. In case of unconsciousness, there’s a risk of aspiration.
- MAOIs: Combining MAOIs with certain opioids can lead to severe adverse reactions, including agitation, seizures, and even death.
- MXE: MXE can potentiate opioid effects but also increase the risk of respiratory depression and organ toxicity.
- Nitrous: Nitrous and dextropropoxyphene can potentiate ataxia and sedation, potentially causing loss of consciousness. Aspiration due to vomiting is a risk.
- PCP: PCP may reduce opioid tolerance, increasing the risk of overdose.
- Tramadol: Combining tramadol with dextropropoxyphene increases the risk of seizures and may result in central nervous system and respiratory depression.
- Grapefruit: While not psychoactive, grapefruit can affect opioid metabolism, potentially prolonging their effects and increasing toxicity.
Serotonin Syndrome Risk:
Combinations with certain substances can lead to dangerously high serotonin levels, causing serotonin syndrome, a medical emergency. These substances include MAOIs, serotonin releasers (e.g., MDMA), SSRIs, SNRIs, and 5-HTP.
Legal status
- Germany: Dextropropoxyphene falls under the regulations of BtMG Anlage II in Germany, rendering it illegal to manufacture, import, possess, sell, or transfer without a license.
- Russia: Dextropropoxyphene is classified as a Schedule II controlled substance in Russia.
- Switzerland: Dextropropoxyphene is recognized as a controlled substance, listed under Verzeichnis A in Switzerland. Medicinal use is allowed, with certain preparations included in Verzechnis C and others excluded.
- United Kingdom: In the United Kingdom, Dextropropoxyphene is categorized as a Class C substance, falling under either Schedule 2 or Schedule 5, depending on the dosage.
- United States: Dextropropoxyphene is designated as a Schedule II or Schedule IV Controlled Substance in the United States, contingent on the dosage and additional ingredients. It has been discontinued for prescription use in the United States, although some compounding pharmacies may still offer it.
FAQ
1. What is Dextropropoxyphene?
Dextropropoxyphene is a synthetic opioid analgesic (painkiller) that was previously used to treat moderate to severe pain. It was marketed under various brand names, including Darvon and Darvocet.
2. Is Dextropropoxyphene still available on the market?
No, Dextropropoxyphene has been withdrawn from the market in many countries, including the United States, due to safety concerns and a risk of overdose and cardiac toxicity.
3. Why was Dextropropoxyphene withdrawn from the market?
Dextropropoxyphene was withdrawn from the market due to reports of serious cardiac side effects and an increased risk of overdose when taken at higher-than-recommended doses. Its limited analgesic efficacy compared to its potential risks led to its removal from the market.
4. How does Dextropropoxyphene work?
Dextropropoxyphene works by binding to opioid receptors in the brain and spinal cord, which results in a reduction in the perception of pain. It also has a depressant effect on the central nervous system.
5. Are there any alternative medications for pain management?
Yes, there are many alternative medications available for pain management, including other opioid and non-opioid analgesics, as well as non-pharmacological approaches like physical therapy, acupuncture, and relaxation techniques. Your healthcare provider can help determine the most appropriate treatment for your pain.
6. What were the common side effects of Dextropropoxyphene?
Common side effects of Dextropropoxyphene include dizziness, drowsiness, nausea, constipation, and respiratory depression. However, its use was associated with more severe side effects, such as cardiac arrhythmias.
7. Can Dextropropoxyphene be addictive?
Yes, like many opioid medications, Dextropropoxyphene has the potential for abuse and addiction if used improperly or for extended periods. It should only be used under the guidance of a healthcare professional.
8. Can I take Dextropropoxyphene with other medications?
It’s essential to inform your healthcare provider about all the medications you are taking to avoid potential drug interactions. Dextropropoxyphene could interact with other drugs, including alcohol and central nervous system depressants, leading to serious health risks.
9. Is Dextropropoxyphene available as a generic medication?
Generic versions of Dextropropoxyphene were available before it was withdrawn from the market in many countries. However, these generic formulations are also no longer available in most places.
10. What should I do if I have unused Dextropropoxyphene medication?
If you have unused Dextropropoxyphene medication, it is advisable to dispose of it safely and according to local regulations. Do not share it with others, and do not keep it for future use, especially if it has been discontinued in your region.
11. Can I still get Dextropropoxyphene through special authorization or prescription?
In most countries, Dextropropoxyphene has been banned, and it is not available for prescription use. Check with your healthcare provider for alternative pain management options.
12. Where can I get more information about Dextropropoxyphene?
For more information about Dextropropoxyphene or any other medication, consult your healthcare provider or pharmacist. Additionally, you can refer to official drug information resources provided by your country’s health department or regulatory agency.
References
- Nicotinic Receptor Blockade: A study conducted by Xiao et al. (2001) revealed that methadone, its metabolites, and structural analogs can block the function of rat α3β4 nicotinic receptors. This interaction highlights the potential for complex neurochemical effects when combining methadone with other depressants[^1^].
- Opioid Toxidrome and Grapefruit Juice: Ershad et al. (2020) reported a case of opioid toxidrome following the consumption of grapefruit juice in the context of methadone maintenance. This unexpected interaction suggests that grapefruit juice may potentiate the effects of methadone, leading to adverse reactions[^2^].
- Serotonin Toxicity: Gillman (2005) discussed the risks associated with combining monoamine oxidase inhibitors (MAOIs) with opioid analgesics. Such combinations can potentially result in serotonin toxicity, a life-threatening condition characterized by altered mental status, autonomic dysfunction, and neuromuscular abnormalities[^3^].
- Legal Regulations: It’s essential to be aware of the legal regulations surrounding depressants. In Germany, for example, Anlage II BtMG lists controlled substances, including depressants, subject to strict regulations[^4^]. Similarly, the Russian Federation has governmental regulations (Постановление Правительства РФ) concerning the control of substances with depressant properties[^5^].
- Swiss Federal Chancellery Regulations: The Swiss Federal Chancellery regulates narcotic substances, psychotropic substances, precursor chemicals, and auxiliary chemicals. Understanding these regulations is crucial to complying with the law and ensuring safety[^6^].
- DEA Controlled Drugs: The United States Drug Enforcement Administration (DEA) maintains schedules of controlled substances, including depressants. Familiarize yourself with the DEA schedules to understand the legal status of specific depressants[^7^].