Beautiful Plants For Your Interior
Summary
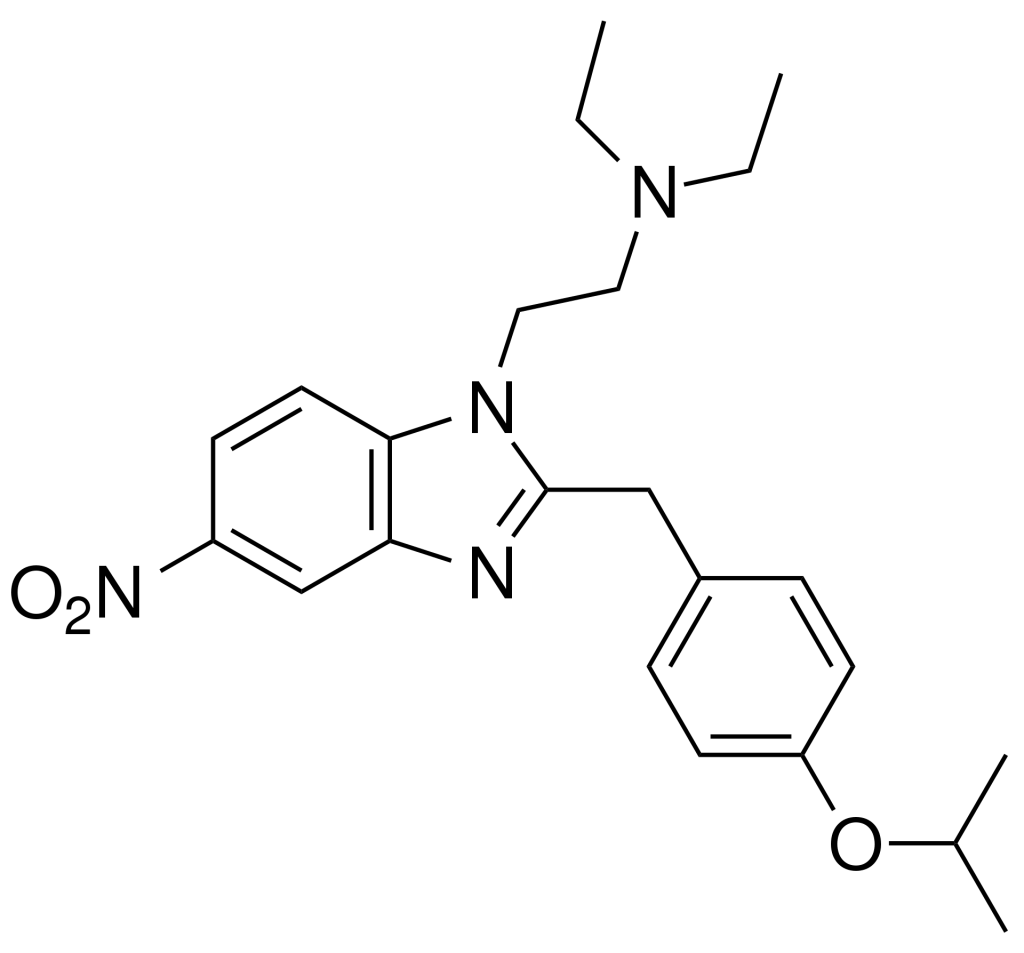
Isotonitazene is an opioid analgesic drug derived from benzimidazole, and it shares a relationship with etonitazene. Marketed as a designer drug, it exhibits approximately half the potency of etonitazene in animal studies. However, it’s likely even less potent in humans, mirroring the pattern observed with etonitazene (which was approximately 1000 times as potent as morphine in animal models but only about 60 times as rich in humans).
In November 2019, researchers fully characterized isotonitazene, obtaining it from an online vendor. In their paper, the authors conducted a comprehensive analytical structure elucidation and determined its potency at the μ-opioid receptor using an in vitro biological functional assay. Although the assay didn’t directly compare isotonitazene to morphine, it was found to be approximately 2.5 times more potent than hydromorphone and slightly more potent than fentanyl.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 14188-81-9 |
|---|---|
| PubChem CID | 145721979 |
| ChemSpider | 73962294 |
| UNII | ZFY1ZBQ8AV |
| Chemical and physical data | |
| Formula | C23H30N4O3 |
| Molar mass | 410.518 g·mol−1 |
Side effects
The side effects associated with benzimidazole-derived opioids are expected to resemble those of fentanyl. These may encompass symptoms such as itching, nausea, and potentially severe respiratory depression, which can pose life-threatening risks.[citation needed]
Isotonitazene has been identified in multiple fatalities in Europe since March 2019 and in the United States since August 2019. NPS Discovery, the Center for Forensic Science Research and Education, and NMS Labs reported these findings.
Legal Status:
In response to these concerns, the U.S. Drug Enforcement Administration (DEA) issued a notice of intent to issue a temporary order scheduling isotonitazene in Schedule I of the Controlled Substances Act. This regulation came into effect on August 20, 2020.
FAQ
1. What is Isotonitazene?
Isotonitazene is a synthetic opioid analgesic drug that belongs to the benzimidazole class of compounds. It shares a relationship with etonitazene and is sometimes marketed as a designer drug.
2. What are the potential side effects of Isotonitazene?
The side effects of Isotonitazene are likely to be similar to those of fentanyl, a potent opioid. These side effects may include itching, nausea, and potentially severe respiratory depression, which can be life-threatening.
3. When was Isotonitazene first detected in fatalities?
Isotonitazene was first detected in fatalities in Europe in March 2019 and in the United States since August 2019. These findings were reported by various organizations and laboratories specializing in forensic analysis.
4. Is Isotonitazene a legally controlled substance?
Yes, in response to safety concerns, the U.S. Drug Enforcement Administration (DEA) issued a notice of intent to schedule Isotonitazene as a controlled substance. This scheduling placed it in Schedule I of the Controlled Substances Act, and it came into effect on August 20, 2020.
5. What are the risks associated with Isotonitazene use?
Isotonitazene use poses significant risks due to its potent opioid nature. These risks include overdose, respiratory depression, addiction, and potential fatality. It is crucial to be aware of these risks and exercise caution.
6. Is there any legitimate medical use for Isotonitazene?
Isotonitazene is not approved for medical use and is not prescribed by healthcare professionals. It is considered a designer drug and is not intended for any legitimate medical applications.
7. Where can I find more information about Isotonitazene and its risks?
Seek information from reputable sources such as government health agencies, addiction treatment centres, and substance abuse hotlines. It’s advisable to consult with a healthcare professional if you have concerns about Isotonitazene or its effects.
References
1. Riksdagsförvaltningen. “Förordning (1992:1554) om kontroll av narkotika Svensk författningssamling 1992:1992:1554 t.o.m. SFS 2021:301 – Riksdagen”. You can find this information on the official website of the Riksdagen (in Swedish).
2. Blanckaert P, Cannaert A, Van Uytfanghe K, Hulpia F, Deconinck E, Van Calenbergh S, Stove C (April 2020). “Report on a novel emerging class of highly potent benzimidazole NPS opioids: Chemical and in vitro functional characterization of isotonitazene.” The study was published in Drug Testing and Analysis, Volume 12, Issue 4, Pages 422–430. DOI: 10.1002/dta.2738. PMID: 31743619. S2CID: 208187034.
3. Casy AF, Wright J (October 1966). “Ionisation constants and partition coefficients of some analgesically active 2-benzylbenzimidazole derivatives and related compounds.” This research was published in The Journal of Pharmacy and Pharmacology, Volume 18, Issue 10, Pages 677–683. DOI: 10.1111/j.2042-7158.1966.tb07782.x. PMID: 4382076. S2CID: 41516830.
4. Ujváry, István; Christie, Rachel; Evans-Brown, Michael; Gallegos, Ana; Jorge, Rita; de Morais, Joanna; Sedefov, Roumen (March 2021). “DARK Classics in Chemical Neuroscience: Etonitazene and Related Benzimidazoles.” Published in ACS Chemical Neuroscience, Volume 12, Issue 7, Pages 1072–1092. DOI: 10.1021/acschemneuro.1c00037. PMID: 33760580. S2CID: 232356192.
5. Power, Mike (13 March 2020). “40 Americans Are Dying Every Month from Taking This New, Legal Opioid.” Published by Vice.
6. Zagorski CM, Myslinski JM, Hill LG (September 2020). “Isotonitazene as a contaminant of concern in the illegal opioid supply: A practical synthesis and cost perspective.” Published in The International Journal on Drug Policy, Volume 86, Page 102939. DOI: 10.1016/j.drugpo.2020.102939. PMID: 32977186. S2CID: 221939376.
7. Critical Review Report: Isotonitazene [PDF]. This report was issued by the Expert Committee on Drug Dependence during its Forty-third Meeting. It took place in Geneva from October 12 to 20, 2020. The report provides information on Isotonitazene.
8. Hunger A, Kebrle J, Rossi A, Hoffmann K (October 1957). “[Synthesis of analgesically active benzimidazole derivatives with basic substitutions]” [Synthesis of analgesically active benzimidazole derivatives with basic substitutions]. The study was published in Experientia, Volume 13, Issue 10, Pages 400–401. DOI: 10.1007/BF02161116. PMID: 13473817. S2CID: 32179439.
9. Rossi A, Hunger A, Kebrle J, Hoffmann K (1960). “Benzimidazol-Derivate und verwandte Heterocyclen. IV. Die Kondensation von o-Phenylendiamin mit α-Aryl- und γ-Aryl-acetessigester” [Benzimidazole derivatives and related heterocycles IV. The condensation of o-phenylenediamine with α-aryl and γ-aryl-acetoacetate]. This research was published in Helvetica Chimica Acta (in German), Volume 43, Issue 4, Pages 1046–1056. DOI: 10.1002/hlca.19600430413.
10. Rossi A, Hunger A, Kebrle J, Hoffmann K (1960). “Benzimidazol-Derivate und verwandte Heterocyclen V. Die Kondensation von o-Phenylendiamin mit aliphatischen und alicyclicischen β-Ketoestern” [Benzimidazole derivatives and related heterocycles V. The condensation of o-phenylenediamine with aliphatic and alicyclic β-keto esters]. This research was published in Helvetica Chimica Acta (in German), Volume 43, Issue 5, Pages 1298–1313. DOI: 10.1002/hlca.19600430515.
11. Rossi A, Hunger A, Kebrle J, Hoffmann K (1960). “Benzimidazol-Derivate und verwandte Heterocyclen VI. Synthese von Phenyl-[1-aminoalkyl-benzimidazolyl-(2)]-essigsäure-estern und -amiden” [Benzimidazole derivatives and related Heterocycles VI. Synthesis of phenyl-[1-aminoalkyl-benzimidazolyl-(2)]-acetic acid esters and amides]. This research was published in Helvetica Chimica Acta (in German), Volume 43, Issue 6, Pages 1727–1733. DOI: 10.1002/hlca.19600430634.
12. Gross F, Turrian H (October 1957). “[Benzimidazole derivatives with strong analgesic effects]” [Benzimidazole derivatives with strong analgesic effects]. This research was published in Experientia, Volume 13, Issue 10, Pages 401–403. DOI: 10.1007/BF02161117. PMID