Beautiful Plants For Your Interior
Summary
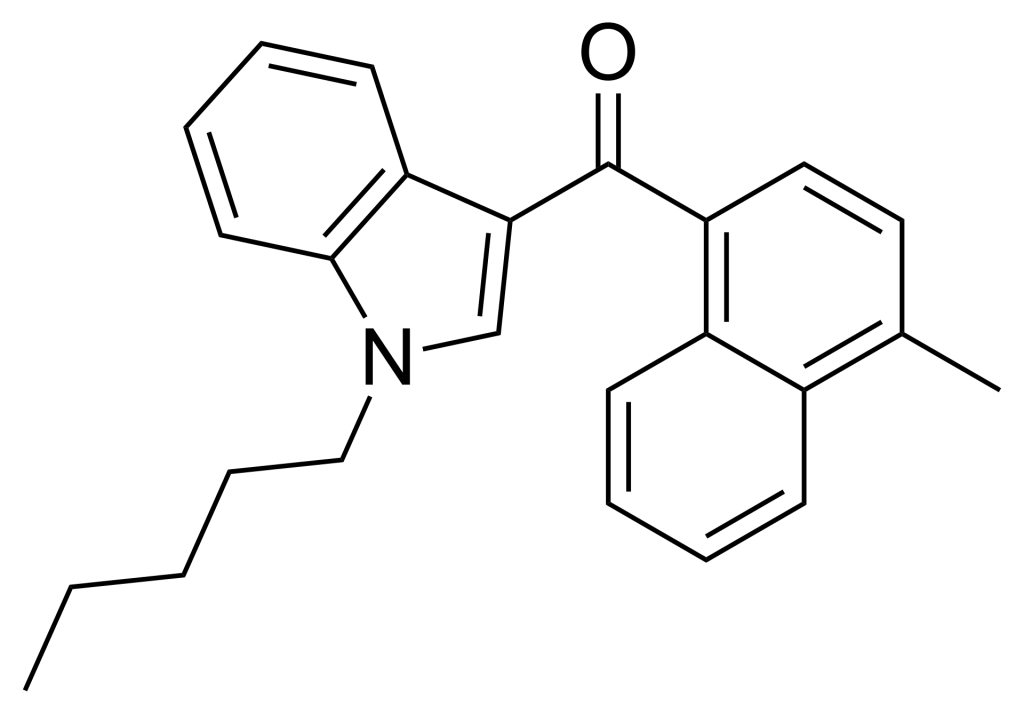
JWH-122, a synthetic cannabimimetic compound, was first identified by John W. Huffman. This compound is a methylated derivative of JWH-018 and exhibits a Ki of 0.69 nM at CB1 and 1.2 nM at CB2 receptors.
In January 2015, a concerning incident occurred when more than 40 individuals reportedly fell ill after consuming Rosca de Reyes, a traditional holiday bread purchased from a bakery in Santa Ana, California. It was discovered that this bread had been adulterated with JWH-122, raising health and safety concerns.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 619294-47-2 |
|---|---|
| ChemSpider | 24623066 |
| UNII | 44147RI31X |
| ChEMBL | ChEMBL557004 |
| CompTox Dashboard (EPA) | DTXSID00210965 |
| Chemical and physical data | |
| Formula | C25H25NO |
| Molar mass | 355.481 g·mol−1 |
Legal status
Australia:
In Australia, JWH-122 is classified as a Schedule 9 prohibited substance under the Poisons Standard as of October 2015. A Schedule 9 substance is one with the potential for misuse or abuse, making its manufacture, possession, sale, or use illegal, except when specifically required for medical, scientific research or approved purposes such as analysis, education, or training with the authorization of Commonwealth and State or Territory Health Authorities.
China:
As of October 2015, JWH-122 is regulated as a controlled substance in China.
United States:
Within the United States, JWH-122 is categorized as a Schedule I Controlled Substance. This classification signifies its status as an illegal substance under federal law.
FAQ
1. What is JWH-122?
JWH-122 is a synthetic compound classified as a synthetic cannabinoid receptor agonist. It’s known for its interactions with cannabinoid receptors in the body, and it’s a derivative of JWH-018.
2. Is JWH-122 legal in Australia?
No, JWH-122 is considered a Schedule 9 prohibited substance in Australia as per the Poisons Standard (October 2015). This classification makes its manufacture, possession, sale, or use illegal, with limited exceptions for specific research and analytical purposes, with proper authorization.
3. What is the status of JWH-122 in China?
In China, JWH-122 is a controlled substance as of October 2015. This means it is subject to regulation, and its use may be restricted or prohibited.
4. How is JWH-122 classified in the United States?
JWH-122 is categorized as a Schedule I Controlled Substance in the United States. This classification indicates that it is illegal at the federal level, making its possession, sale, or use prohibited.
5. What are the potential effects of JWH-122?
JWH-122’s effects can be similar to those of natural cannabinoids, including altered mood, relaxation, and changes in perception. However, synthetic cannabinoids like JWH-122 may have unpredictable and harmful side effects.
6. Is JWH-122 used for medical purposes?
JWH-122 has not been approved for any medical use. Its safety and efficacy for medical applications have not been established through rigorous testing and research. For medical conditions, it is essential to consult healthcare professionals and use approved medications.
7. Are there risks associated with JWH-122 use?
Yes, risks are associated with JWH-122, including potential health risks and legal consequences. The use of synthetic cannabinoids can lead to unpredictable and harmful side effects, making it unsafe and discouraged.
8. Where can I find more information about JWH-122?
To learn more about JWH-122, it is advisable to consult reputable scientific literature and healthcare professionals. Always prioritize safety and well-being and adhere to local laws and regulations regarding controlled substances.
References
- Anvisa’s Regulatory Control (2023-07-24): Anvisa, the Brazilian Health Regulatory Agency, issued RDC Nº 804, which outlines a list of substances under special control in Brazilian Portuguese. This list encompasses narcotic, psychotropic, precursor, and other substances. The resolution was published in the Diário Oficial da União on July 25, 2023, and archived for reference. This regulatory update is significant in terms of controlling specific substances in Brazil.
- Research on Cannabinoid Receptor Agonists (January 2005): Research conducted by Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, and others in January 2005 delves into the structure-activity relationships of 1-alkyl-3-(1-naphthoyl)indoles concerning the cannabinoid CB(1) and CB(2) receptors. The study explores the steric and electronic effects of naphthoyl substituents and the development of highly selective CB(2) receptor agonists. This research contributes to our understanding of synthetic cannabinoids and their interactions with specific receptors.
- Synthetic Drug Contamination (February 2, 2015): In February 2015, a concerning incident was reported in which holiday bread from a California bakery was contaminated with a synthetic drug. This incident, highlighted by Food Safety News, underscores the importance of monitoring food safety and the potential risks associated with the inadvertent presence of synthetic substances in food products.
- Australian Poisons Standard (October 2015): The Australian Government’s Poisons Standard, dated October 2015, plays a crucial role in regulating substances. It categorizes and controls substances, particularly those with the potential for misuse or harm, emphasizing safety and control.
- Chinese Regulations (September 2015): In September 2015, the China Food and Drug Administration issued a notice regarding the management of non-medical narcotic and psychotropic substances. This highlights China’s regulatory measures to control these substances to ensure safety and compliance.
- Controlled Substances in the U.S. (DEA): The U.S. Department of Justice’s Drug Enforcement Division (DEA) oversees controlled substances in the United States. This authoritative source provides information on substances that are strictly regulated due to their potential for misuse or abuse, ensuring legal control and safety.