Beautiful Plants For Your Interior
Summary
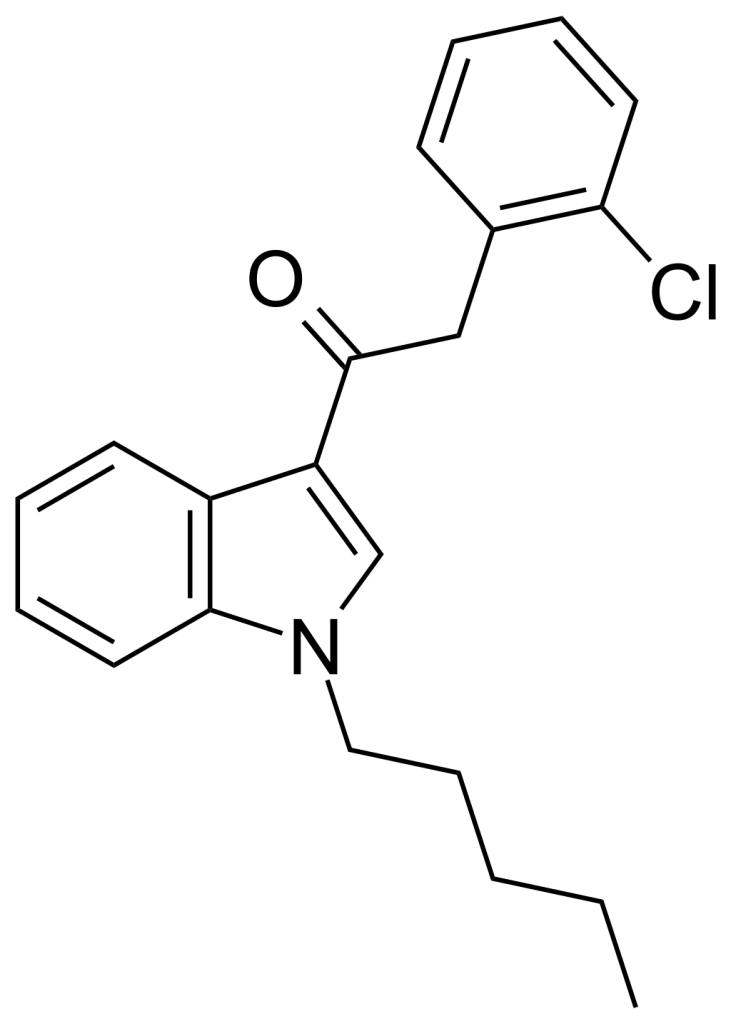
JWH-203, also known as 1-pentyl-3-(2-chlorophenylacetyl)indole, is a member of the phenylacetylindole family and serves as an analgesic chemical. This compound functions as a cannabinoid agonist, demonstrating nearly equal affinity for CB1 and CB2 receptors, with a Ki of approximately 8.0 nM at CB1 and 7.0 nM at CB2. Originally named after its discoverer, John W. Huffman, it has, at times, been illicitly marketed as an ingredient in synthetic cannabis smoking blends without his consent.
Regarding its structure, JWH-203 shares similarities with related compounds like the 2′-methoxy compound JWH-250, the 2′-bromo compound JWH-249, and the 2′-methyl compound JWH-251. What sets JWH-203 apart is the presence of a phenyl acetyl group instead of the naphthyl ring typically found in most aminoalkyl indole cannabinoid compounds. It is noteworthy that JWH-203 exhibits the highest in vitro binding affinity for cannabinoid receptors among compounds within the phenylacetyl group.
Interestingly, despite having a weaker CB1 Ki value in vitro, the 2-methylindole derivative JWH-204 has proven to be more potent than JWH-203 in animal tests assessing cannabinoid activity, although it remains less powerful than JWH-249.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 864445-54-5 (JWH-203) 864445-55-6 (JWH-204) |
|---|---|
| PubChem CID | 44397500 |
| ChemSpider | 23256082 |
| UNII | 52CP80V8FY |
| CompTox Dashboard (EPA) | DTXSID00235557 |
| ECHA InfoCard | 100.233.381 |
| Chemical and physical data | |
| Formula | C21H22ClNO |
| Molar mass | 339.86 g·mol−1 |
Legal status
In the United States, JWH-203 is classified as a Schedule I Controlled Substance.
Since October 2015, JWH-203 has been regulated as a controlled substance in China.
FAQ
1. What is JWH-203?
JWH-203 is a chemical compound from the phenylacetylindole family, known for its cannabinoid agonist properties. It interacts with cannabinoid receptors in the body.
2. How does JWH-203 work?
JWH-203 functions as a cannabinoid agonist, binding to and activating cannabinoid receptors, specifically CB1 and CB2.
3. What is its affinity for CB1 and CB2 receptors?
JWH-203 demonstrates nearly equal affinity for CB1 and CB2 receptors, with Ki values of around 8.0 nM at CB1 and 7.0 nM at CB2.
4. Who discovered JWH-203, and why is it notable?
JWH-203 was initially discovered by John W. Huffman. It gained notoriety as it has been illicitly marketed without his permission as an ingredient in synthetic cannabis smoking blends.
5. What distinguishes JWH-203 from other synthetic cannabinoids?
JWH-203 is unique due to the presence of a phenyl acetyl group in its chemical structure, setting it apart from aminoalkyl indole cannabinoid compounds with naphthyl rings.
6. Is JWH-203 legal to use in the United States?
No, JWH-203 is classified as a Schedule I Controlled Substance in the United States, making its manufacture, possession, or distribution illegal.
7. How is JWH-203 regulated in China?
Since October 2015, JWH-203 has been regulated as a controlled substance in China.
8. Are there similar compounds to JWH-203?
Yes, there are related compounds, such as JWH-204, JWH-250, and JWH-249, which belong to the same family of synthetic cannabinoids.
9. What are the potential effects and risks associated with JWH-203 use?
Effects and risks may vary, but synthetic cannabinoids like JWH-203 can lead to altered perception, relaxation, and anxiety. Adverse effects can also occur, and the safety of its use is not well-established.
10. Is JWH-203 used for medical purposes?
JWH-203 is not approved for any medical use and is primarily associated with recreational or illicit use.
11. Where can I find more information about JWH-203?
You can explore scientific literature, drug regulation sources, and reputable educational websites for more information about JWH-203. Always ensure that your sources are credible and reliable when seeking information about such substances.
References
- “Ustawa z dnia 15 kwietnia 2011 r. o zmianie ustawy o przeciwdziałaniu narkomanii ( Dz.U. 2011 nr 105 poz. 614 )”. Accessible via Internetowy System Aktów Prawnych. Retrieved on 17 June 2011.
- Bononi M, Belgi P, and Tateo F. (July 2011). “Providing Analytical Insights: Data for the Identification of the Cannabimimetic Phenylacetylindole JWH-203” (PDF). Published in the Journal of Analytical Toxicology, Volume 35, Issue 6, Pages 360–363. DOI: 10.1093/anatox/35.6.360. PMID: 21740693.
- Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, et al. (September 2005). “New Frontiers Explored: 1-Pentyl-3-phenylacetylindoles – A Novel Class of Cannabimimetic Indoles”. Published in Bioorganic & Medicinal Chemistry Letters, Volume 15, Issue 18, Pages 4110–4113. DOI: 10.1016/j.bmcl.2005.06.008. PMID: 16005223.
- Manera C, Tuccinardi T, and Martinelli A. (April 2008). “Insights into Indoles: Indoles and Related Compounds as Cannabinoid Ligands”. Published in Mini Reviews in Medicinal Chemistry, Volume 8, Issue 4, Pages 370–387. DOI: 10.2174/138955708783955935. PMID: 18473928.
- Bononi M, Belgi P, and Tateo F. (July 2011). “Analytical Data for the Identification of the Cannabimimetic Phenylacetylindole JWH-203” (PDF). Published in the Journal of Analytical Toxicology, Volume 35, Issue 6, Pages 360–363. DOI: 10.1093/anatox/35.6.360. PMID: 21740693.
- Wiley JL, Marusich JA, Martin BR, and Huffman JW. (June 2012). “Common Ground: 1-Pentyl-3-phenylacetylindoles and JWH-018 Share Similar In Vivo Cannabinoid Profiles in Mice”. Published in Drug and Alcohol Dependence, Volume 123, Issues 1–3, Pages 148–153. DOI: 10.1016/j.drugalcdep.2011.11.001. PMC 3294131. PMID: 22127210.
- “Controlled Substances” (PDF). Issued by the Drug Enforcement Administration.
- “关于印发《非药用类麻醉药品和精神药品列管办法》的通知” (in Chinese). China Food and Drug Administration. Dated 27 September 2015. Archived from the original on 1 October 2015. Retrieved on 1 October 2015.