Beautiful Plants For Your Interior
Summary
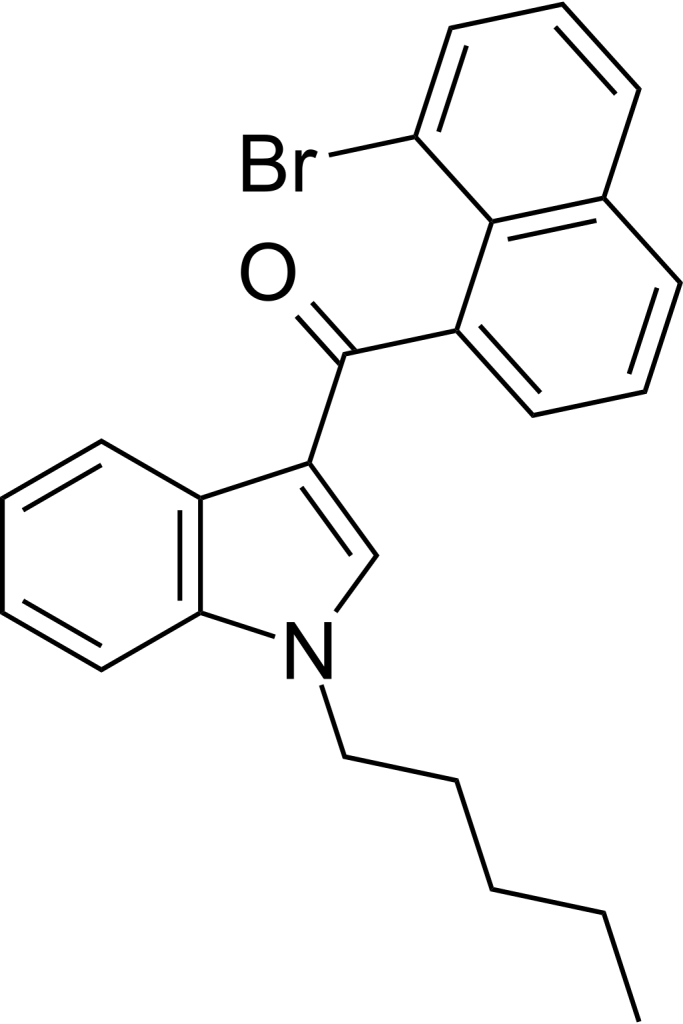
JWH-424 is a member of the naphthoylindole family, characterized by its role as a cannabinoid agonist targeting both the CB1 and CB2 receptors. However, it demonstrates moderate selectivity for the CB2 receptor, with a Ki value of 5.44nM at CB2 in contrast to 20.9 nM at CB1. The heavier 8-iodo analog of this compound exhibits even greater selectivity for CB2, especially when compared to its 2-methyl derivative, which boasts a remarkable 40-fold preference for CB2. On the other hand, the 1-propyl homologs in this series display considerably lower affinity at both receptors, indicating a reduced overall association for the 8-substituted naphthoylindoles.
In the United States, JWH-424, like other CB1 receptor agonists belonging to the 3-(1-naphthyl)indole class, is categorized as a Schedule I Controlled Substance.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1366068-04-3 |
|---|---|
| PubChem CID | 57458937 |
| ChemSpider | 28645324 |
| UNII | O4Q3AIP6BJ |
| CompTox Dashboard (EPA) | DTXSID50159855 |
| Chemical and physical data | |
| Formula | C24H22BrNO |
| Molar mass | 420.350 g·mol−1 |
FAQ
1. What is JWH-424?
JWH-424 is a synthetic compound from the naphthoylindole family, acting as a cannabinoid agonist targeting both the CB1 and CB2 receptors.
2. How does JWH-424 interact with cannabinoid receptors?
JWH-424 interacts with cannabinoid receptors, specifically CB1 and CB2. Its selectivity for CB2 is moderate, making it an attractive compound for research.
3. What is the selectivity of JWH-424 for CB1 and CB2 receptors?
JWH-424 exhibits moderate selectivity for the CB2 receptor with a Ki value of 5.44nM at CB2 compared to 20.9 nM at CB1. This indicates a preference for CB2, although it still interacts with CB1.
4. Are there related compounds with different selectivity?
Yes, related compounds, such as the heavier 8-iodo analog, can display even greater selectivity for CB2. The 2-methyl derivative of JWH-424, for example, exhibits a remarkable 40-fold preference for CB2.
5. How do 1-propyl homologs in this compound series behave?
The 1-propyl homologs in this compound series generally show lower affinity at CB1 and CB2 receptors, indicating a reduced overall association for the 8-substituted naphthoylindoles.
6. What is the legal status of JWH-424 in the United States?
JWH-424, like other CB1 receptor agonists of the 3-(1-naphthyl)indole class, is classified as a Schedule I Controlled Substance in the United States, subject to strict legal control.
7. What is the significance of JWH-424’s receptor selectivity in research?
JWH-424’s distinct receptor selectivity makes it a valuable tool for cannabinoid research, providing insights into the interactions and potential therapeutic applications of compounds targeting CB1 and CB2 receptors.
References
- Exploring Naphthoylindole Compounds (2012): A study conducted by Wiley JL, Smith VJ, Chen J, Martin BR, and Huffman JW in March 2012, titled “Synthesis and pharmacology of 1-alkyl-3-(1-naphthoyl)indoles: steric and electronic effects of 4- and 8-halogenated naphthoyl substituents,” delves into the synthesis and pharmacological properties of 1-alkyl-3-(1-naphthoyl)indoles. This research highlights the impact of steric and electronic factors related to 4- and 8-halogenated naphthoyl substituents.
- Legal Regulations on Controlled Substances: The United States Code, Title 21, Section 812 (21 U.S.C. § 812) outlines the scheduling of controlled substances, providing the legal framework for the classification and control of various substances in the United States. This code is a vital reference for understanding the legal status of specific compounds and their regulation.