Beautiful Plants For Your Interior
Where to Buy MEAI Online – Trusted Sources for Purchasing MEAI
If you are looking to buy MEAI online, it’s essential to find reputable sources that offer high-quality products. Numerous vendors sell MEAI, but not all of them are reliable. Make sure to choose MEAI vendors that provide detailed product descriptions, secure payment options, and discreet shipping. Whether you’re located in the USA, Canada, or internationally, it’s crucial to find a trustworthy MEAI shop that guarantees product authenticity.
How to Order MEAI – A Step-by-Step Guide for Secure Purchases
Ordering MEAI online can be a straightforward process, but it’s important to follow certain steps to ensure a safe transaction. Start by finding a reputable MEAI vendor who ships to your country, whether you’re in the USA, Canada, or elsewhere. Verify that the vendor has positive reviews and offers secure payment methods. Once you’ve selected a trusted MEAI shop, proceed to place your order, keeping in mind that some vendors may offer special discounts or bulk deals for larger quantities.
MEAI for Sale – Best Deals and Promotions
For those interested in MEAI for sale, there are often various promotions and discounts available. Look for limited-time offers or special deals on bulk purchases. Many MEAI vendors offer competitive pricing, so it’s a good idea to compare different sellers to ensure you get the best value for your money. Some vendors also offer first-time customer discounts or free shipping, making it even more affordable to buy MEAI online.
Summary
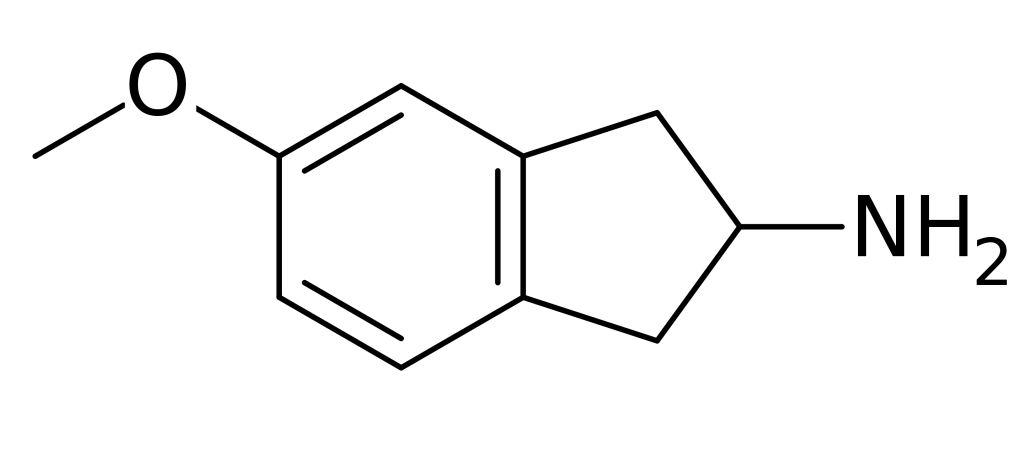
MEAI, also known as 5-methoxy-2-aminoindane, 5-MeO-AI, or Chaperon, belongs to the family of indane molecules. Its molecular structure made its initial, albeit implicit, appearance within a patent document back in 1998. Subsequently, it was explicitly and pharmacologically described in a peer-reviewed paper in 2017 by David Nutt and Ezekiel Golan, among others. Further insights into MEAI’s pharmacokinetics, pharmacodynamics, and metabolism were provided in another article published in February 2018 by Shimshoni, David Nutt, Ezekiel Golan, and their colleagues. A year later, MEAI was the subject of yet another peer-reviewed study conducted by Halberstadt and colleagues.
The aminoindane family of compounds, to which MEAI belongs, traces its initial chemical description back to 1980.
MEAI played an early role as a candidate for alcohol replacement drugs, emerging during a late 2010s movement to find less-toxic alternatives to alcohol, which British psychopharmacologist David Nutt spearheaded. This movement had a ripple effect across Europe as well[10].
In an act of gonzo journalism, Michael Slezak, a writer for New Scientist, documented his experience with MEAI after being provided with it by Dr. Zee (Ezekiel Golan) following an interview. Golan claimed to have invented MEAI and initially intended it for sale as a legal high but later expressed plans to collaborate with David Nutt and his company, DrugScience, to develop further MEAI based on Golan’s patents as a “binge behavior regulator” and an “alcoholic beverage substitute.”
In 2018, a company called the Diet Alcohol Corporation of the Americas (DACOA) openly marketed a MEAI-based drink named “Pace” for sale in the USA and Canada. Pace was described as a 50ml bottle containing 160mg of MEAI dissolved in mineral water. Distribution ceased after Health Canada issued a warning, citing the substance’s structural similarity to amphetamine as a reason it was considered illegal to market for consumption in Canada. In a December 2018 interview with CBC News, Ezekiel Golan (Dr. Z/Dr. Zee) publicly identified himself as the “lead scientist” behind Pace and claimed that “tens of thousands” of bottles had already been sold in Canada. Golan asserted that the MEAI in Pace was “manufactured in India” and “bottled in Delaware.” Health Canada, according to CBC News, declared, “Pace is an illegal and unauthorized product in Canada.”
Various media outlets, including Chemistry World and the BBC, have referred to Ezekiel Golan as “the man who invents legal highs.” The Guardian also dubbed him “the godfather of legal highs” for his role in reintroducing substituted cathinone-based drugs, commonly sold as bath salts, including Mephedrone.
On May 26, 2022, preparations were made for MEAI’s registration with the FDA by Clearmind Medicine Inc. Clearmind Medicine claimed substantial intellectual property holdings based on Ezekiel Golan’s patents. In March 2022, Clearmind Medicine announced supportive evidence from animal studies in mice that demonstrated a suppression of alcohol consumption. In June 2022, they revealed promising results from animal studies suggesting potential benefits in treating cocaine addiction with MEAI.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 73305-09-6 |
|---|---|
| PubChem CID | 12147687 |
| ChemSpider | 11591017 |
| UNII | AD8S15863A |
| CompTox Dashboard (EPA) | DTXSID80478566 |
| Chemical and physical data | |
| Formula | C10H13NO |
| Molar mass | 163.220 g·mol−1 |
FAQ
1. What is MEAI?
MEAI, or 5-methoxy-2-aminoindane, is a chemical compound belonging to the indane family of molecules. It has been explored for its potential effects and applications.
2. How was MEAI first described or discovered?
The molecular structure of MEAI was initially mentioned implicitly within a patent document in 1998. Subsequent explicit and pharmacological descriptions appeared in peer-reviewed papers in 2017 and 2018 by researchers including David Nutt and Ezekiel Golan.
3. What is the aminoindane family of molecules?
The aminoindane family consists of compounds with structural features similar to MEAI. A shared indane core structure characterizes these molecules.
4. What role did MEAI play in the movement to replace alcohol with less-toxic alternatives?
MEAI was an early candidate for alcohol replacement drugs during a movement in the late 2010s to find safer alternatives to alcohol. This initiative, championed by British psychopharmacologist David Nutt, had an impact across Europe.
5. What research or experiences have been documented with MEAI?
MEAI has been the subject of scientific research, including studies on its pharmacokinetics, pharmacodynamics, and potential effects. Journalists and writers have also reported on their experiences with MEAI.
6. Who is Ezekiel Golan (Dr. Z/Dr. Zee) in relation to MEAI?
Ezekiel Golan is associated with the development and promotion of MEAI. He has expressed intentions to collaborate with David Nutt and DrugScience to develop MEAI as a “binge behavior regulator” and an “alcoholic beverage substitute.”
7. Why did the marketing of the MEAI-based drink “Pace” face issues in Canada?
The marketing of “Pace” was halted in Canada after Health Canada raised concerns about its structural similarity to amphetamine. As a result, it was considered illegal for consumption in Canada.
8. What are the implications of MEAI’s registration with the FDA by Clearmind Medicine Inc.?
Clearmind Medicine Inc. has taken steps to register MEAI with the FDA. They claim significant intellectual property holdings based on Ezekiel Golan’s patents and are exploring potential applications, including the suppression of alcohol consumption and the treatment of cocaine addiction.
9. Is MEAI legal and safe for use?
The legal status and safety of MEAI may vary by region and are subject to evolving regulations. Due to limited research, it’s challenging to make definitive statements about its safety. As with any psychoactive substance, caution is advisable.
10. Where can I find more information about MEAI?
To access reliable information on MEAI and its current status, consult reputable sources, scientific literature, and government health agencies. Staying informed is essential when dealing with substances like MEAI.
References
- US 5708018, Haadsma-Svensson SR, Andersson BR, Sonesson CA, Lin CH, Waters RN, Svensson KA, Carlsson PA, Hansson LO, Stjernlof NP, “2-aminoindans as selective dopamine D3 ligands,” published 1998-01-13, assigned to Pharmacia & Upjohn Co.In 1998, a patent, US 5708018, introduced the concept of “2-aminoindans as selective dopamine D3 ligands,” marking a significant development in the understanding of related compounds.
- Shimshoni JA, Winkler I, Edery N, Golan E, van Wettum R, Nutt D (March 2017). “Toxicological evaluation of 5-methoxy-2-aminoindane (MEAI): Binge mitigating agent in development.” Toxicology and Applied Pharmacology. 319: 59–68.In March 2017, Shimshoni and colleagues conducted a toxicological evaluation of 5-methoxy-2-aminoindane (MEAI), focusing on its potential as a “binge mitigating agent in development.” This research explored MEAI’s safety and effects.
- Shimshoni JA, Sobol E, Golan E, Ben Ari Y, Gal O (March 2018). “Pharmacokinetic and pharmacodynamic evaluation of 5-methoxy-2-aminoindane (MEAI): A new binge-mitigating agent.” Toxicology and Applied Pharmacology. 343: 29–39.In March 2018, Shimshoni and colleagues delved into the pharmacokinetics and pharmacodynamics of 5-methoxy-2-aminoindane (MEAI) as a “new binge-mitigating agent.” This research provided insights into how MEAI interacts with the body.
- Halberstadt AL, Brandt SD, Walther D, Baumann MH (March 2019). “2-Aminoindan and its ring-substituted derivatives interact with plasma membrane monoamine transporters and α2-adrenergic receptors.” Psychopharmacology. 236 (3): 989–999.In March 2019, Halberstadt and colleagues investigated the interactions of 2-aminoindan and its ring-substituted derivatives with plasma membrane monoamine transporters and α2-adrenergic receptors, shedding light on their pharmacological properties.
- Sainsbury PD, Kicman AT, Archer RP, King LA, Braithwaite RA (2011). “Aminoindanes–the next wave of ‘legal highs’?” Drug Testing and Analysis. 3 (7–8): 479–482.In 2011, Sainsbury and colleagues examined aminoindanes as potential “legal highs,” contributing to the understanding of their effects and safety profiles.
- Nutt D (23 October 2013). “Decision making about illegal drugs: time for science to take the lead.” Nobel Forum, Karolinska Institutet – via YouTube.In October 2013, Dr. David Nutt addressed the need for science to inform decision-making regarding illegal drugs, emphasizing the importance of scientific research in this context.
- Nutt DJ, King LA, Phillips LD (November 2010). “Drug harms in the UK: a multicriteria decision analysis.” Lancet. London, England. 376 (9752): 1558–65.In November 2010, a study by Nutt and colleagues assessed the relative harms of various drugs in the UK, emphasizing the importance of considering a range of factors in drug policy decision-making.
- Forster K (24 September 2016). “Hangover free alcohol is finally here.” The Independent.In September 2016, an article discussed the development of “hangover-free” alcohol alternatives, reflecting the ongoing search for less harmful drinking options.
- Wermter B (29 April 2019). “Rauschmittel und gesellschaftliche Probleme” [Drug-related societal issues]. Benedict Wermter (in German).In April 2019, Benedict Wermter delved into the intersection of recreational drugs and societal challenges, highlighting the complexity of the issue.
- Slezak M (30 December 2014). “High and dry? Party drug could target excess drinking.” New Scientist.Michael Slezak explored the potential of party drugs to address excessive drinking, providing insights into the evolving landscape of alcohol alternatives.
- Slezak M (9 August 2014). “An Interview with Dr. Z” (PDF). New Scientist. pp. 1–3.An interview with Dr. Zee (Ezekiel Golan) offered perspectives on the development and intentions behind substances like MEAI, shedding light on the creator’s motivations.
- US 10406123B2, Golan E, “Binge behavior regulators,” issued 2019-09-10A US patent, US 10406123B2, outlines innovations related to “binge behavior regulators,” contributing to the evolving landscape of substances aimed at regulating excessive consumption.
- US 20170360067, Golan E, “Alcoholic beverage substitutes,” issued 2017-12-21A patent, US 20170360067, discusses the creation of “alcoholic beverage substitutes,” reflecting ongoing efforts to develop alternatives to traditional alcoholic drinks.
- “Advisory – Health Canada warns consumers that Pace, promoted as an alcohol substitute, is unauthorized and may pose serious health risks.” Health Canada. 21 December 2018 – via CISION.Health Canada issued a warning about “Pace,” an MEAI-based drink promoted as an alcohol substitute, highlighting potential health risks and unauthorized marketing.
- Brunet J (24 April 2019). “FACT CHECK: Is Pace, an “Alcohol Alternative,” Legal in Canada?”. The Walrus. Toronto, Ontario.An investigation into the legal status of “Pace,” an MEAI-based “alcohol alternative,” provided clarity on its regulatory standing in Canada.
- Wright J (8 December 2018). “Is this drink really a new ‘alcohol alternative’?”. Information Morning Saint John.An exploration of a drink touted as a “new alcohol alternative” raised questions about its potential as a substitute for traditional alcoholic beverages.
- Extance A (6 September 2017). “The rising tide of ‘legal highs’.” Chemistry World.“The rising tide of ‘legal highs'” by Alexhander Extance delved into the emergence of legal highs, shedding light on the evolution of psychoactive substances.
- “Meet Dr. Zee – the man who invented legal highs”. BBC. 23 January 2018.A BBC feature introduced Dr. Zee (Ezekiel Golan) as a key figure in the creation of legal highs, providing insights into his role in the field.
- Jonze T (24 May 2016). “Dr Zee, the godfather of legal highs: ‘I test everything on myself'”. TheGuardian.com.The Guardian’s profile of Dr. Zee (Ezekiel Golan) portrayed him as a central figure in the development of legal highs, emphasizing his hands-on approach to testing these substances.
- “Clearmind Medicine”. www.clearmindmedicine.com.Clearmind Medicine’s website offers information about their initiatives and research, including the development of substances like MEAI.
- “Clearmind Medicine Inc”. CSE:CMND.Clearmind Medicine Inc.’s listing on the Canadian Securities Exchange (CSE) provides insights into their role in the field of psychoactive substances.
- וינרב, גלי (16 February 2022). “החברה שמנסה להפוך סם פסיכדלי למוצר נגד התמכרות” [The company trying to turn a psychedelic drug into an anti-addiction product]. Globes (in Hebrew).An article in Hebrew discusses a company’s efforts to transform a psychedelic substance into an anti-addiction product, reflecting evolving research and applications in the field.
- US 10137096, Golan E, “Binge behavior regulators,” published 2018-11-27A US patent, US 10137096, outlines innovations in “binge behavior regulators,” contributing to the development of substances aimed at regulating excessive consumption.
- EP 3230256, Golan E, “Alcoholic beverage substitutes,” published 2019-11-13A European patent, EP 3230256, details the creation of “alcoholic beverage substitutes,” reflecting international efforts to develop alternatives to traditional alcoholic drinks.
- EP 3230255, Golan E, “Binge behavior regulators,” published 2017-10-18A European patent, EP 3230255, discusses “binge behavior regulators,” contributing to the evolving landscape of substances aimed at regulating excessive consumption.
- “The Science and IP Behind our Treatments”. Clearmind.Clearmind provides insights into the science and intellectual property behind their treatment initiatives, including substances like MEAI.
- “Clearmind Medicine”. www.clearmindmedicine.com. Retrieved 14 August 2022.Clearmind Medicine’s website offers resources and updates related to their research and initiatives in the field of psychoactive substances.
- “Clearmind Medicine”. www.clearmindmedicine.com. Retrieved 2022-10-16.Clearmind Medicine’s website provides access to information about their research and developments, allowing for updates on their work.