Beautiful Plants For Your Interior
Summary
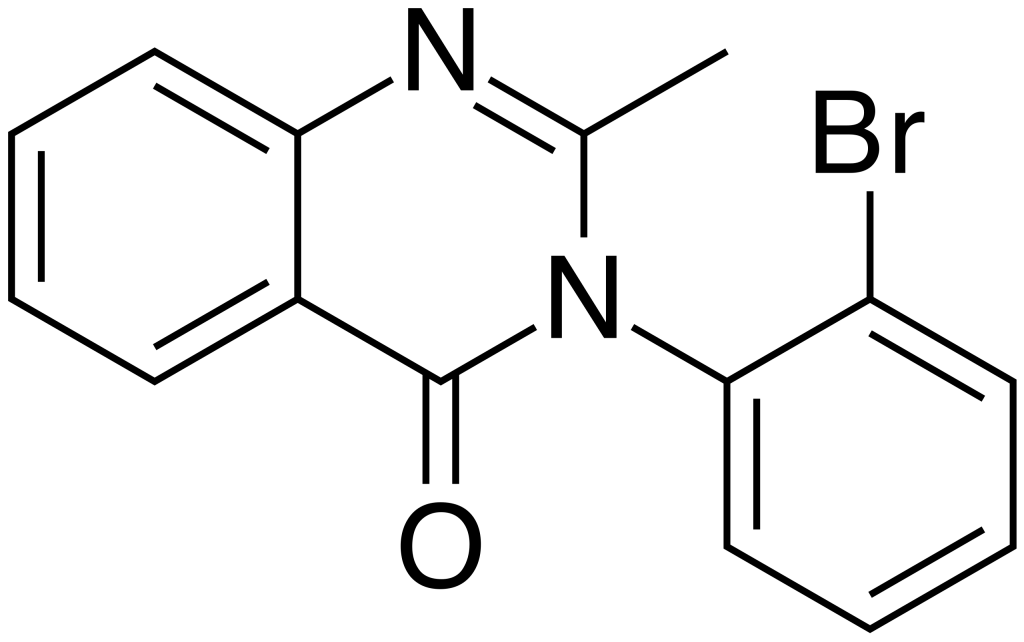
Mebroqualone (often abbreviated as MBQ) belongs to the quinazolinone class of compounds and is recognized as a GABAergic substance. It serves as an analog of mecloqualone, sharing comparable sedative and hypnotic properties with its parent compound, owing to its agonistic effects at the β subtype of the GABA receptor. The synthesis of Mebroqualone dates back to the 1960s.
One distinctive feature of Mebroqualone is the substitution of a bromine atom for the chlorine atom found on the 3-phenyl ring, setting it apart from mecloqualone. Notably, in 1998, Germany enacted legislation to prohibit the legal use and distribution of Mebroqualone. Unfortunately, limited information is available about this compound, but it appears that it was traded on the black market in Germany, serving as a designer drug akin to mecloqualone.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 4260-20-2 |
|---|---|
| PubChem CID | 364842 |
| ChemSpider | 323878 |
| UNII | ST66S8F8EY |
| CompTox Dashboard (EPA) | DTXSID20327105 |
| Chemical and physical data | |
| Formula | C15H11BrN2O |
| Molar mass | 315.170 g·mol−1 |
FAQ
1. What is Mebroqualone?
Mebroqualone, often abbreviated as MBQ, is a chemical compound belonging to the quinazolinone class. It is considered a GABAergic substance and serves as an analog of mecloqualone.
2. What are the primary properties and effects of Mebroqualone?
Mebroqualone shares sedative and hypnotic properties with its parent compound, mecloqualone. These effects are attributed to its ability to act as an agonist at the β subtype of the GABA receptor. It is used primarily for its calming and sleep-inducing qualities.
3. When was Mebroqualone initially synthesized?
Mebroqualone was first synthesized in the 1960s.
4. What distinguishes Mebroqualone from Mecloqualone?
One key distinction is the substitution of a bromine atom in Mebroqualone for the chlorine atom found on the 3-phenyl ring in mecloqualone. This subtle structural change sets the two compounds apart.
5. Is Mebroqualone legal in any country?
As of my last knowledge update in January 2022, Mebroqualone was made illegal in Germany in 1998. Its legal status in other countries may vary, so it’s essential to research and understand the specific regulations and laws regarding its use and possession in your region.
6. Is Mebroqualone safe to use?
The safety of Mebroqualone is a subject of concern, especially in recreational contexts. Limited information is available, making it essential to be cautious and aware of potential risks. Using substances like Mebroqualone should be done responsibly, if at all.
7. Can Mebroqualone still be obtained on the black market or as a designer drug?
While the legal status of Mebroqualone may restrict its availability, it may still be obtained on the black market in some regions, sometimes marketed as a designer drug. However, the purchase and use of such substances can be risky and illegal.
8. Are there any medical or therapeutic uses for Mebroqualone?
Mebroqualone is not recognized for any approved medical or therapeutic uses. It is primarily known for its soothing and hypnotic effects.
9. What precautions should be taken when dealing with substances like Mebroqualone?
It is essential to exercise caution, conduct thorough research, and adhere to local laws and regulations. Using unknown or unregulated substances can pose significant health risks, including the potential for adverse effects and legal consequences.
10. Where can I find more information about Mebroqualone?
Due to its limited documented use and potential risks, it is advisable to rely on reputable sources and consult with healthcare professionals when seeking information or guidance related to Mebroqualone or other similar substances.
References
- In September 1960, Jackman GB, Petrow V, and Stephenson O conducted research focused on “Some 2, 3-disubstituted 3H-4-quinazolones and 3H-4-thioquinazolones.” Their findings were published in The Journal of Pharmacy and Pharmacology, Volume 12, spanning pages 529–538. This publication can be accessed via the DOI: 10.1111/j.2042-7158.1960.tb12705.x and the PMID: 14406263. Additionally, it is indexed with S2CID: 31254238.
- On October 7, 1998, the “Zwölfte Verordnung zur Änderung betäubungsmittelrechtlicher Vorschriften” (Twelfth Regulation Amending Narcotic Drug Regulations) was published in the Bundesgesetzblatt (Federal Law Gazette), Volume I (68), on page 3126. This regulation brought about changes in narcotic drug laws.