Beautiful Plants For Your Interior
Summary
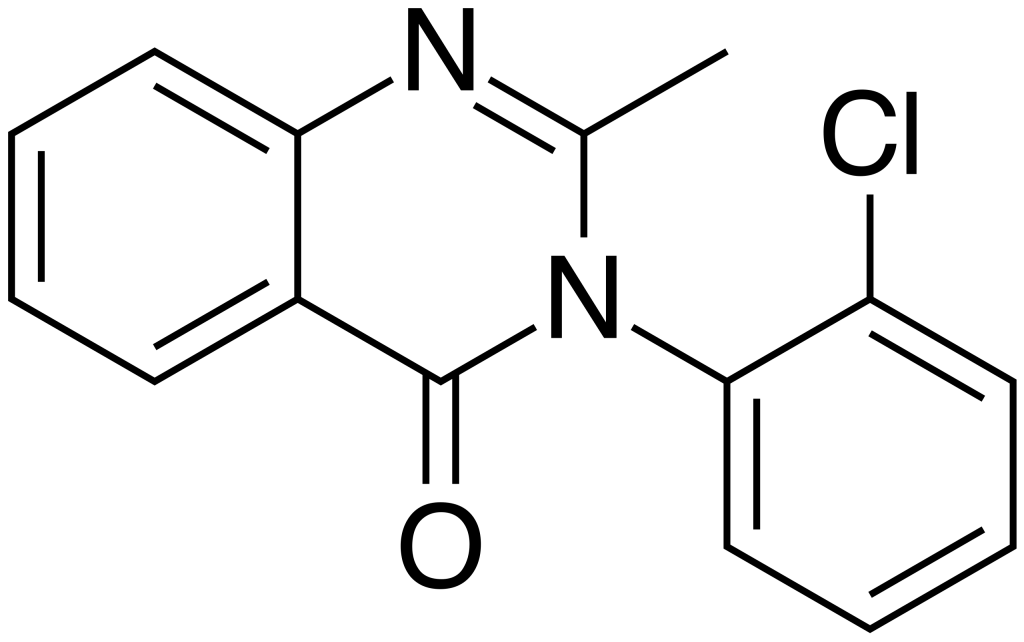
Mecloqualone, also known by trade names such as Nubarene and Casfen, belongs to the Quinazolinone class of compounds. It is categorized as a GABAergic substance and serves as an analog of methaqualone. This compound was first synthesized in 1960 and primarily found its market in France and several other European countries. Mecloqualone exhibits notable properties, including sedative, hypnotic, and anxiolytic effects, which result from its ability to act as an agonist at the β subtype of the GABA receptor. It was initially employed for the treatment of insomnia.
In comparison to methaqualone, Mecloqualone is faster-acting but has a shorter duration of action. Consequently, it was primarily utilized as a sleep aid, distinguishing it from methaqualone, which had a broader application as a general-purpose anxiolytic. Despite its potential, Mecloqualone has yet to achieve the same widespread use as methaqualone. Today, it is no longer prescribed due to concerns regarding its potential for abuse and overdose.
In the United States, Mecloqualone is classified as a Schedule I non-narcotic depressant controlled substance, and its production is regulated, with an annual aggregate manufacturing quota set at 30 grams.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 340-57-8 |
|---|---|
| PubChem CID | 9567 |
| ChemSpider | 9192 |
| UNII | 09XU4VDV7E |
| KEGG | D04877 |
| ChEMBL | ChEMBL279960 |
| CompTox Dashboard (EPA) | DTXSID7048875 |
| ECHA InfoCard | 100.005.848 |
| Chemical and physical data | |
| Formula | C15H11ClN2O |
| Molar mass | 270.714 g·mol−1 |
FAQ
1. What is Mecloqualone?
Mecloqualone is a chemical compound belonging to the Quinazolinone class. It is recognized as a GABAergic substance and serves as an analog of Methaqualone.
2. What are the main properties and effects of Mecloqualone?
Mecloqualone is known for its sedative, hypnotic, and anxiolytic effects. These properties are attributed to its agonistic activity at the β subtype of the GABA receptor. It was primarily used for the treatment of insomnia.
3. When was Mecloqualone first synthesized?
Mecloqualone was initially synthesized in the year 1960.
4. How does Mecloqualone compare to Methaqualone?
Mecloqualone is faster-acting but has a shorter duration of action compared to Methaqualone. As a result, it was primarily employed as a sleep aid, in contrast to Methaqualone, which had a broader application as a general-purpose anxiolytic.
5. Why is Mecloqualone no longer prescribed?
Mecloqualone is no longer prescribed due to concerns about its potential for abuse and overdose. These safety and regulatory concerns have led to its discontinuation as a prescribed medication.
6. Is Mecloqualone regulated by law in any country?
Yes, in the United States, Mecloqualone is classified as a Schedule I non-narcotic depressant controlled substance. This classification makes it subject to strict regulations and controls.
7. Can Mecloqualone still be found or used today?
The availability and use of Mecloqualone have significantly declined. It is no longer prescribed, and its production and distribution are restricted due to its controlled status in many countries. It is not recommended for use due to safety concerns.
8. What are the risks associated with Mecloqualone use?
The risks of Mecloqualone use include the potential for abuse, addiction, and overdose. Its controlled status reflects the recognition of these risks, making it an unsafe substance for recreational or medicinal use.
9. Is there any medical or therapeutic use for Mecloqualone today?
Mecloqualone is not recognized for any approved medical or therapeutic use. It is considered a substance with a high potential for abuse and adverse effects.
10. Where can I find more information about Mecloqualone and its history?
To learn more about Mecloqualone and its historical context, you can refer to scientific literature, medical archives, and authoritative sources on pharmacology and controlled substances. It’s essential to rely on credible information sources when seeking further details about this compound.
References
- On July 24, 2023, Anvisa issued “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” (Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control). This regulatory document is available in Brazilian Portuguese and was published in the Diário Oficial da União on July 25, 2023. An archived version is accessible from August 27, 2023.
- In September 1960, Jackman GB, Petrow V, and Stephenson O conducted research on “Some 2, 3-disubstituted 3H-4-quinazolones and 3H-4-thioquinazolones.” Their findings were published in The Journal of Pharmacy and Pharmacology, Volume 12, spanning pages 529–538. This publication can be accessed via the DOI: 10.1111/j.2042-7158.1960.tb12705.x and the PMID: 14406263. Additionally, it is indexed with S2CID: 31254238.
- In 1963, Mouren P, Giraud F, and Pinsard N explored the “[Clinical use of a new psycholeptic: Mecloqualone]” in the Marseille Medical journal. Their research delved into the clinical application of Mecloqualone, with the publication located at volume 100, pages 599–602. This work can be referenced using the PMID: 13936358.
- In November 1969, Dubnk B, Towne CA, and Bush MT conducted a study on the “Detection, assay, and rate of excretion of mecloqualone in animals and man.” This research was published in Toxicology and Applied Pharmacology, Volume 15, Issue 3, with pages 632–641. The publication is available via the DOI: 10.1016/0041-008X(69)90065-9 and the PMID: 5353825.
- Information regarding “Established Aggregate Production Quotas for Schedule I and II Controlled Substances and Assessment of Annual Needs for the List I Chemicals Ephedrine, Pseudoephedrine, and Phenylpropanolamine for 2022” is available but may require a more specific source or reference for further details.