Beautiful Plants For Your Interior
Summary
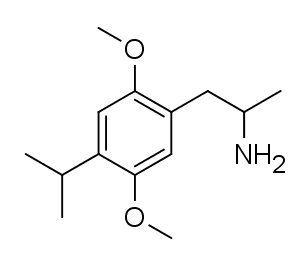
2,5-Dimethoxy-4-isopropylamphetamine also recognized as DOiP and DOiPr, is classified as a psychedelic substance belonging to the phenethylamine and amphetamine chemical groups. The pioneering synthesis of this compound was attributed to Alexander Shulgin and was documented in his book PiHKAL (Phenethylamines I Have Known And Loved). Shulgin noted that DOiPR demonstrated significantly lower potency than DOPr, necessitating doses of 20–30 mg to induce discernible alterations in the mental state. However, limited information is available regarding the pharmacological attributes, metabolic pathways, and potential toxicity of DOiPR.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 42306-96-7 53581-56-9 (hydrochloride salt) |
|---|---|
| PubChem CID | 44265275 |
| ChemSpider | 23108724 |
| UNII | PF0P8RBU0X |
| Chemical and physical data | |
| Formula | C14H23NO2 |
| Molar mass | 237.343 g·mol−1 |
FAQ
- What is 2,5-Dimethoxy-4-isopropylamphetamine (DOiP)? 2,5-Dimethoxy-4-isopropylamphetamine (DOiP) is a psychedelic compound belonging to the phenethylamine and amphetamine classes known for its hallucinogenic properties.
- Who discovered DOiP? DOiP was first synthesized by the eminent chemist Alexander Shulgin, whose extensive work in psychoactive substances is documented in his book PiHKAL (Phenethylamines i Have Known And Loved).
- What are the effects of DOiP? DOiP is known to induce alterations in perception, mood, and thought processes, typical of other psychedelics in its class.
- Is DOiP used for any medical purposes? DOiP is primarily used in scientific research and is not approved for any medical applications. Its use outside of controlled research settings can be hazardous.
- Are there any known safety concerns associated with DOiP use? Limited data is available regarding the pharmacological properties and potential toxicity of DOiP, highlighting the need for caution and further research regarding its safety profile.
- How does the potency of DOiP compare to other similar compounds? According to Shulgin’s observations, DOiP exhibits notably lower potency than certain related substances, necessitating comparatively higher doses for the desired effects.
- Is DOiP a controlled substance in any country? Regulations concerning DOiP vary by country. While some nations may have specific restrictions on its use, others may not have formal laws pertaining to DOiP.
References
- Glennon RA, Seggel MR (November 1989). Exploring the Interplay of Phenylisopropylamines with Central 5-HT2 Receptors: Insights from Quantitative Structure-Activity Relationships. This research delves into the intricate relationship between phenylisopropylamines and central 5-HT2 receptors, offering valuable insights. (pp. 264–280). doi:10.1021/bk-1989-0413.ch018. ISBN 978-0-8412-1702-7.
- Seggel MR, Yousif MY, Lyon RA, Titeler M, Roth BL, Suba EA, Glennon RA (March 1990). A Comprehensive Structure-Affinity Study: Probing the Binding of 4-Substituted Analogues of 1-(2,5-dimethoxyphenyl)-2-aminopropane at 5-HT2 Serotonin Receptors. This study investigates the binding characteristics of 4-substituted analogs and their interaction with 5-HT2 serotonin receptors. (Journal of Medicinal Chemistry, 33(3), 1032–6). doi:10.1021/jm00165a023. PMID 2308135.
- Aldous FA, Barrass BC, Brewster K, Buxton DA, Green DM, Pinder RM, et al. (October 1974). Unraveling Structure-Activity Relationships in Psychotomimetic Phenylalkylamines. This exploration focuses on the complex interplay of structure and activity in psychotomimetic phenylalkylamines. (Journal of Medicinal Chemistry, 17(10), 1100–11). doi:10.1021/jm00256a016. PMID 4418757.
- Shulgin A, Shulgin A (September 1991). PiHKAL: A Chemical Love Story #71 DOPR. In this insightful book, Alexander Shulgin discusses DOPR (1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane), providing valuable information about this compound. (Transform Press, p. 978). Retrieved on June 27, 2015.