Beautiful Plants For Your Interior
Summary
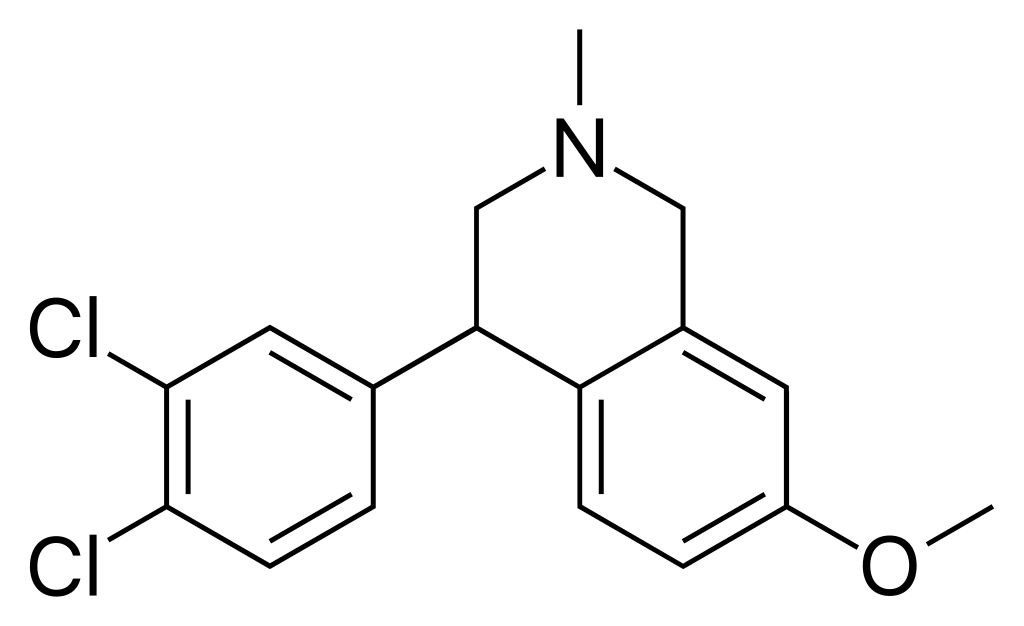
Diclofensine (Ro 8-4650) emerged from Hoffmann-La Roche’s research efforts in the 1970s aimed at discovering a novel antidepressant. Subsequent investigations revealed that the active component was the (S)-isomer. This compound functions as a stimulant by operating as a triple monoamine reuptake inhibitor, primarily impeding the reuptake of dopamine and norepinephrine, exhibiting binding affinities (Ki) of 16.8 nM, 15.7 nM and 51 nM for DAT, NET, and SERT (dopamine, norepinephrine, and serotonin transporters), respectively. It demonstrated its effectiveness as an antidepressant during human trials, displaying a relatively limited incidence of side effects. Yet, its clinical development was eventually halted, possibly due to concerns regarding its potential for misuse.
Chemically, Diclofensine belongs to the tetrahydroisoquinoline (THIQ) derivative class, akin to nomifensine.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 67165-56-4 |
|---|---|
| PubChem CID | 68871 |
| ChemSpider | 62103 |
| UNII | 09HKW863J6 |
| ChEMBL | ChEMBL287257 |
| Chemical and physical data | |
| Formula | C17H17Cl2NO |
| Molar mass | 322.23 g·mol−1 |
Synthesis
The synthesis of diclofensine (6) begins with the condensation of m-anisaldehyde 591-31-1 with methylamine, yielding N-methyl-3-methoxybenzenemethanimine [16928-30-6]. Subsequent reduction of this Schiff-base intermediate with sodium borohydride produces (3-methoxybenzyl)methylamine 41789-95-1. Alkylation of compound (2) with 3,4-dichlorophenacylbromide 2632-10-2 results in the formation of CID:59580342 (4). The next step involves reducing the benzoyl ketone to obtain alcohol 802051-24-7. Finally, an acid-catalyzed intramolecular cyclization process completes the synthesis, resulting in the formation of diclofensine (6), a 4-aryl-THIQ derivative.

FAQ
- What is diclofensine?
- Diclofensine is a compound that was initially developed as an antidepressant. It belongs to a class of drugs known as stimulants and acts as a triple monoamine reuptake inhibitor, primarily affecting the reuptake of dopamine and norepinephrine.
- How was Diclofensine discovered?
- Hoffmann-La Roche discovered Diclofensine in the 1970s during the search for new antidepressant medications. Researchers found that the (S)-isomer of Diclofensine was responsible for its antidepressant activity.
- How does Diclofensine work?
- Diclofensine works by inhibiting the reuptake of certain neurotransmitters, including dopamine and norepinephrine. This action increases the levels of these neurotransmitters in the brain, which can have mood-altering effects and make it effective as an antidepressant.
- Was diclofensine effective as an antidepressant?
- Yes, Diclofensine was found to be effective as an antidepressant in human trials. It demonstrated the ability to alleviate symptoms of depression with relatively few side effects.
- Why was Diclofensine discontinued?
- Diclofensine was ultimately discontinued from clinical development, possibly due to concerns about its abuse potential. This decision may have been influenced by its stimulant properties and the potential for misuse.
- Is Diclofensine still available for medical use?
- Diclofensine is not available for medical use and is not approved for any specific therapeutic indication. It is considered a discontinued or withdrawn drug.
- Are there any alternatives to Diclofensine for depression treatment?
- Yes, there are various antidepressant medications available today that are approved for the treatment of depression. These include selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and other classes of antidepressants. It is important to consult with a healthcare professional to determine the most appropriate treatment for depression.
- Is Diclofensine used for any other purposes?
- While Diclofensine was primarily developed as an antidepressant, it is not currently used for any therapeutic purpose. It is mainly of historical significance in the field of pharmacology.
- Is Diclofensine a controlled substance?
- Information regarding the legal status of Diclofensine may vary by country, but it is generally not a commonly used or regulated substance due to its discontinued status.
- Is Diclofensine related to any other drugs?
- Diclofensine is chemically related to other tetrahydroisoquinoline (THIQ) derivatives, such as nomifensine. These compounds share structural similarities but may have different pharmacological properties.
References
- U.S. Patent 3947456 A, attributed to Alfred Rheiner, titled “Substituted 4-Phenyl Isoquinolines,” was published on March 30, 1976, and it was assigned to Hoffman-La Roche Inc.
- In his book titled Chirality and the Biological Activity of Drugs, Roger Crossley, in 1995, discussed various aspects of drug chirality. The book was published by CRC Press, Inc., located at 2000 Corporate Blvd., N.W., Boca Raton, Florida 33431, and can be found on page 138. The ISBN for this book is 978-0-8493-9140-8.
- In 1982, Keller HH, Schaffner R, Carruba MO, Burkard WP, Pieri M, Bonetti EP, et al., conducted research on “Diclofensine (Ro 8-4650),” a potent monoamine uptake inhibitor. They compared its biochemical and behavioral effects to nomifensine. This study was published in Advances in Biochemical Psychopharmacology (Vol. 31, pages 249–263) and can be found under PMID 6979165.
- In July 1982, Omer LM conducted pilot trials with diclofensine, a novel psychoactive drug, on depressed patients. This research was published in the International Journal of Clinical Pharmacology, Therapy, and Toxicology (Vol. 20, No. 7, pages 320–326) and can be found under PMID 7107085.
- In 1988, Di Renzo G, Amoroso S, Taglialatela M, Canzoniero LM, Maida P, Lombardi G, and Annunziato L studied pure uptake blockers of dopamine, including diclofensine, and their effects on prolactin secretion. This study was published in Life Sciences (Vol. 42, No. 21, pages 2161–2169) and can be found under doi:10.1016/0024-3205(88)90131-2 and PMID 2968488.
- In May 1986, Gasić S, Korn A, and Eichler HG investigated the effect of diclofensine, a novel antidepressant, on peripheral adrenergic function. This research was published in Clinical Pharmacology and Therapeutics (Vol. 39, No. 5, pages 582–585) and can be found under doi:10.1038/clpt.1986.100 and PMID 3698467.
- In August 1989, Andersen PH explored the selectivity and molecular mechanism of action of the dopamine inhibitor GBR 12909. This study was published in the European Journal of Pharmacology (Vol. 166, No. 3, pages 493–504) and can be found under doi:10.1016/0014-2999(89)90363-4 and PMID 2530094.
- In 1981, Cherpillod C and Omer LM conducted a controlled trial with diclofensine, a new psychoactive drug, for the treatment of depression. This trial was published in The Journal of International Medical Research (Vol. 9, No. 5, pages 324–329) and can be found under doi:10.1177/030006058100900505 and PMID 7028532.
- In March 1984, Omer OL, Díaz-Olivera M, and Ismail S conducted research on the therapeutic efficacy and tolerance of diclofensine in psychoreactive depression. They compared it to placebo in a double-blind study. This study was published in Methods and Findings in Experimental and Clinical Pharmacology (Vol. 6, No. 3, pages 147–151) and can be found under PMID 6379345.
- In May 1986, Funke HJ, Holtmann W, Ismail S, Jansen W, Leonhardt KF, Muth H, et al., conducted a double-blind comparison of diclofensine with nomifensine in outpatients with dysphoric mood. This research was published in Pharmacopsychiatry (Vol. 19, No. 3, pages 120–123) and can be found under doi:10.1055/s-2007-1017168 and PMID 3725890.
- In May 1983, Culig J, Ehsanullah RS, Hallett C, Iliopoulou A, Matheson I, and Turner P conducted a clinical pharmacological comparison of diclofensine (Ro 8-4650) with nomifensine and amitriptyline in normal human volunteers. This study was published in the British Journal of Clinical Pharmacology (Vol. 15, No. 5, pages 537–543) and can be found under doi:10.1111/j.1365-2125.1983.tb02087.x and PMC 1427714, PMID 6860528.
- In 1990, Lamb RJ and Griffiths RR conducted self-administration experiments in baboons and studied the discriminative stimulus effects of bupropion, nomifensine, diclofensine, and imipramine in rats. This research was published in Psychopharmacology (Vol. 102, No. 2, pages 183–190) and can be found under doi:10.1007/bf02245920 and PMID 2125734.
- In August 1995, Nakachi N, Kiuchi Y, Inagaki M, Inazu M, Yamazaki Y, and Oguchi K studied the effects of various dopamine uptake inhibitors on striatal extracellular dopamine levels and behaviors in rats. This study was published in the European Journal of Pharmacology (Vol. 281, No. 2, pages 195–203) and can be found under doi:10.1016/0014-2999(95)00246-h and PMID 7589207.
- In 1973, Alfred Rheiner Jr. obtained CH 538477, a patent attributed to Hoffmann La Roche.
- Alfred Rheiner’s U.S. Patent 3,947,456 in 1976 was assigned to Hoffman-La Roche Inc.
- In 2010, Shuang Liu, Bruce F. Molino, and Kassoum Nacro were granted WO 2010132442, which was assigned to Albany Molecular Research, Inc. The relevant information can be found on page column 32.