Summary
5-(2-Aminopropyl)indole, also known as 5-API, 5-IT, or PAL-571, is a compound belonging to the indole and phenethylamine families, and it is known for its empathogenic effects. This substance was initially synthesized by Albert Hofmann in 1962. Since 2011, it has been openly marketed as a designer drug and sold by online vendors for recreational purposes.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 3784-30-3 |
|---|---|
| PubChem CID | 55253543 |
| ChemSpider | 25991467 |
| UNII | W98BOE73HH |
| CompTox Dashboard (EPA) | DTXSID30873562 |
| ECHA InfoCard | 100.236.959 |
| Chemical and physical data | |
| Formula | C11H14N2 |
| Molar mass | 174.247 g·mol−1 |
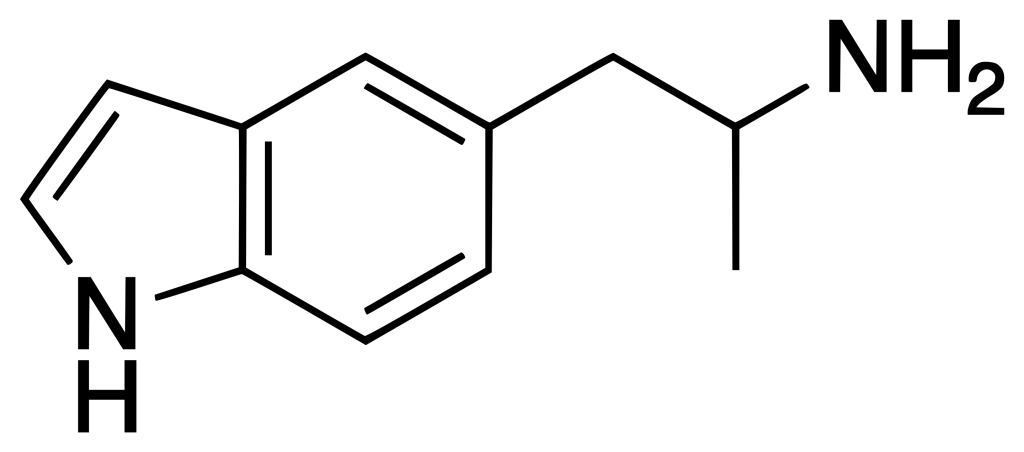
Chemistry
5-IT, despite being a positional isomer of the tryptamine drug αMT, should not be classified as a tryptamine. This distinction arises from the fact that 5-IT substitutes the indole ring at the 5 position rather than the 3 position, aligning it more closely with phenethylamine derivatives like 5-APB in terms of its chemical structure. When employed as a drug, 5-IT is primarily associated with stimulating effects rather than the psychedelic properties commonly linked to tryptamines.
Pharmacology
5-IT functions as a triple monoamine-releasing agent, exhibiting notable potency with EC50 values of 12.9 nM for dopamine, 13.3 nM for norepinephrine, and 104.8 nM for serotonin. Additionally, it acts as an MAO-A inhibitor.
Dosage and effects
Alexander Shulgin provided a concise description of 5-IT in TiHKAL, noting that when taken orally at 20 milligrams, it acts as a prolonged stimulant, resulting in effects such as increased heart rate, reduced appetite, diuresis, and a mild increase in body temperature, lasting for approximately twelve hours. Given that 5-IT falls outside the category of tryptamines, it is not expounded upon in the book beyond this summary.
In cases where 5-IT has been consumed, various symptoms may manifest, including hyperthermia, tachycardia, elevated blood pressure, dilated pupils (mydriasis), restlessness, excessive sweating, jaw clenching, insomnia, disorientation, anxiety, and tremors. It’s important to note that 5-IT functions as a monoamine oxidase inhibitor (MAOI), and when used in combination with contraindicated substances, it can lead to life-threatening consequences, including death.
Deaths
Since its introduction, 5-IT has been associated with 14 fatalities in Sweden. In two instances, 5-IT was the only substance detected in post-mortem analyses, while the remaining twelve cases revealed the presence of other drugs as well. These tragic incidents transpired between April and July 2012, but the definitive identification of 5-IT in post-mortem samples did not occur until July. The victims were all young males aged between 20 and 30. Additionally, eleven non-lethal poisonings attributable to 5-IT were reported during the same timeframe.
Legality
5-IT is a positional isomer of αMT, and as such, it falls under the same legal classification as αMT according to the Controlled Substance Act in the United States. (The Federal Analog Act also incorporates considerations of the substance’s effects.)
In June 2013, 5-IT was prohibited as a temporary class drug alongside nine other related compounds. On March 5, 2014, the UK Home Office declared that 5-API would be designated as a class B drug starting on June 10, 2014, along with all other benzofuran entactogens and several structurally related substances
5-IT is subject to the Australian analog act, recognized as an analog of MDA, characterized by the replacement of up to 2 carbocyclic or heterocyclic ring structures with different carbocyclic or heterocyclic ring structures.
While a formal application for the illegalization of 5-IT in Sweden was submitted on July 26, 2012, it took little effect.
Denmark prohibited 5-IT on September 30, 2012.
In Germany, 5-IT is classified as an Anlage I controlled substance.
The European Commission proposed a decision calling upon its member states to implement measures for the control of 5-(2-aminopropyl)indole. This proposal urged member states to introduce regulatory measures and legal penalties in accordance with their national legislation concerning psychotropic substances.
FAQ
1. What is 5-IT?
5-IT, also known as PAL-571 and 5-Indolyl-2-Aminopropane (5-API), is a psychoactive compound belonging to the indole and phenethylamine chemical classes. It’s recognized for its empathogenic effects and has been available as a designer drug.
2. How does 5-IT affect the body?
5-IT acts as a triple monoamine-releasing agent and exhibits a high affinity for norepinephrine reuptake inhibition. It also has binding affinity for various receptors, including TAAR1, Alpha-2A adrenergic receptors, 5-HT1A, and 5-HT2A receptors.
3. What are the effects of 5-IT when used as a drug?
When consumed, 5-IT is associated with stimulating effects rather than typical psychedelic experiences. Users may encounter symptoms such as hyperthermia, tachycardia, dilated pupils, agitation, excessive sweating, jaw clenching, insomnia, disorientation, restlessness, anxiety, and tremors.
4. Is 5-IT legally regulated?
The legal status of 5-IT varies by country. In the United States, it is considered legally the same as αMT under the Controlled Substance Act. In the UK, it was classified as a class B drug in 2014, along with other benzofuran entactogens and related compounds.
5. Are there any health risks associated with 5-IT?
Yes, there are health risks linked to the use of 5-IT. It is essential to be aware of potential adverse effects, especially when combined with contraindicated substances. In some cases, severe health complications and even fatalities have been reported in connection with 5-IT use.
6. Is 5-IT banned in specific countries?
Yes, 5-IT has been prohibited in several countries, including Denmark, Germany, and Australia. Legal restrictions may vary, so it is crucial to understand the regulations in your specific location.
7. Is there ongoing research or discussion about 5-IT?
The legal status and research on 5-IT may continue to evolve. Some countries and international organizations may be considering further control measures or restrictions on this substance. Stay informed about any updates regarding its legal status in your region.
8. What should I do if I suspect someone has consumed 5-IT and is experiencing adverse effects?
If you suspect someone has ingested 5-IT and is experiencing adverse effects, seek immediate medical attention. Provide as much information as possible about the substance consumed to assist healthcare professionals in providing appropriate care.
9. Is 5-IT considered a controlled substance?
Yes, in many countries, 5-IT is classified as a controlled substance due to its potential for misuse and associated health risks. Legal penalties may apply to its possession, sale, or use.
10. Where can I find more information about 5-IT?
For further information on 5-IT, it is advisable to consult with local drug enforcement agencies, healthcare professionals, or government websites that provide details on controlled substances and current regulations. Always prioritize safety and adhere to local laws and guidelines.
References
- Banks ML, Bauer CT, Blough BE, Rothman RB, Partilla JS, Baumann MH, Negus SS (June 2014). “Abuse-related effects of dual dopamine/serotonin releasers with varying potency to release norepinephrine in male rats and rhesus monkeys.” This study examined the effects of certain compounds on abuse-related behaviors in rats and rhesus monkeys.
- FR 1344579, Hofmann, Albert; Troxler, Franz, “Nouveaux derives de l’indole et leur preparation.” This is a patent document by Albert Hofmann and Franz Troxler discussing new derivatives of indole and their preparation.
- Katselou M, Papoutsis I, Nikolaou P, Spiliopoulou C, Athanaselis S (January 2015). “5-(2-aminopropyl)indole: a new player in the drama of ‘legal highs’ alerts the community.” This paper discusses the emergence of 5-(2-aminopropyl)indole in the context of “legal highs” and community alerts.
- Marusich JA, Antonazzo KR, Blough BE, Brandt SD, Kavanagh PV, Partilla JS, Baumann MH (February 2016). “The new psychoactive substances 5-(2-aminopropyl)indole (5-IT) and 6-(2-aminopropyl)indole (6-IT) interact with monoamine transporters in brain tissue.” This study explores the interaction of 5-IT and 6-IT with monoamine transporters in brain tissue.
- Herraiz T, Brandt SD (July–August 2014). “5-(2-Aminopropyl)indole (5-IT): a psychoactive substance used for recreational purposes is an inhibitor of human monoamine oxidase (MAO).” This paper investigates 5-(2-aminopropyl)indole (5-IT) and its inhibitory effects on human monoamine oxidase (MAO).
- Shulgin, Alexander (December 1997). Tihkal: A Continuation [Paperback]. This book by Alexander Shulgin contains information on various psychoactive substances, possibly including 5-IT.
- “Nätdrog dödade 14 unga män” (in Swedish). Aftonbladet. This Swedish article reports on the deaths of 14 young men related to an internet-sold drug.
- Seetohul LN, Maskell PD, De Paoli G, Pounder DJ (August 2012). “Deaths associated with new designer drug 5-IT” (PDF). This article discusses deaths associated with the designer drug 5-IT.
- “Fem nya ämnen klassas som narkotika” (in Swedish). The Swedish National Institute of Public Health. This Swedish source reports on the classification of new substances, including 5-IT, as narcotics.
- “Temporary class drug order report on 5-6APB and NBOMe compounds.” A report from the UK Home Office regarding temporary class drug orders, including those related to compounds like 5-IT.
- UK Home Office (2014-03-05). “The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014.” This UK government document discusses amendments to the Misuse of Drugs Act 1971, which could impact the legal status of certain substances.
- “Criminal Code Act 1995” (PDF). Australian Government. This document from the Australian Government outlines legal provisions, including those concerning controlled substances.
- “COM(2013) 436 final” (PDF). European Commission. This document from the European Commission addresses control measures and penalties for psychotropic substances in member states.