Summary
5F-APINACA, also recognized as 5F-AKB-48 or 5F-AKB48, is a synthetic cannabinoid based on the indazole structure and has been available for purchase as a designer drug online. In terms of its chemical structure, it closely resembles cannabinoid compounds outlined in patent WO 2003/035005, differing by the presence of a 5-fluorophenyl chain on the indazole 1-position. Interestingly, 5F-APINACA falls under the claims of this patent despite not being explicitly disclosed as an example. This is due to its striking similarity to the pentane nitrile and 4-chlorobutyl compounds described in examples 3 and 4 in the patent.
5F-APINACA was initially identified in South Korea. It is anticipated to exhibit strong agonistic activity towards both the CB1 and CB2 receptors, as suggested by available research. Furthermore, the metabolism of 5F-APINACA has been documented in scientific literature.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1400742-13-3 |
|---|---|
| PubChem CID | 71711119 |
| ChemSpider | 29339965 |
| UNII | TX64ZY5P0R |
| CompTox Dashboard (EPA) | DTXSID80856800 |
| Chemical and physical data | |
| Formula | C23H30FN3O |
| Molar mass | 383.511 g·mol−1 |
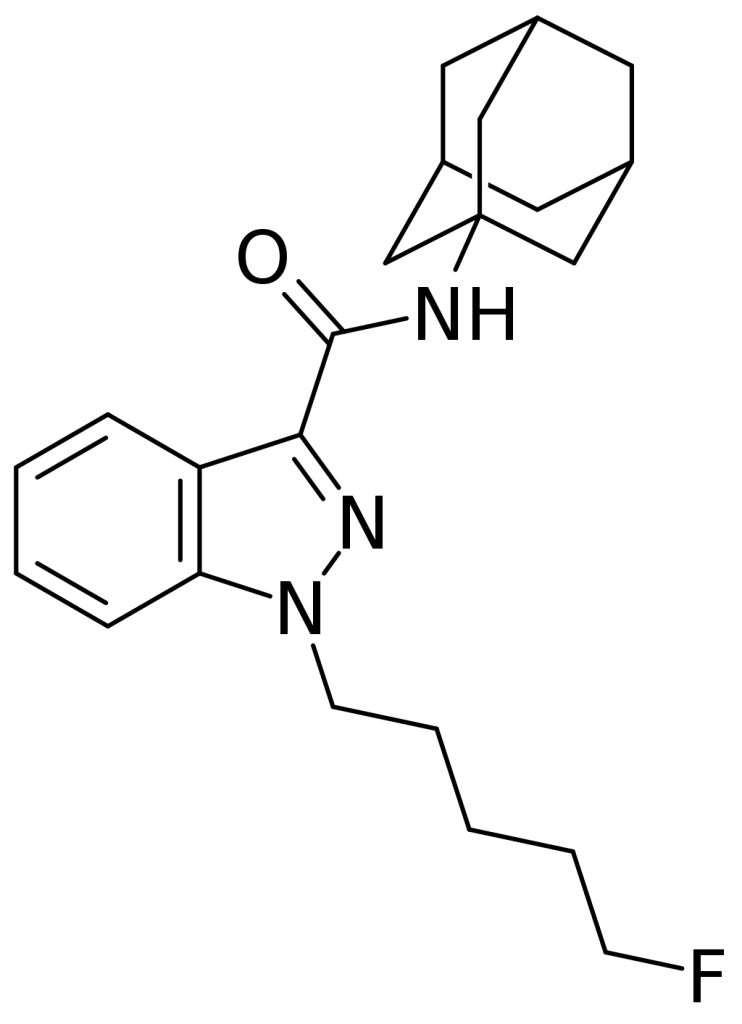
Pharmacology
5F-APINACA functions as a full agonist with a remarkable binding affinity, demonstrating 1.94 nM at CB1 and 0.266 nM at CB2 cannabinoid receptors.
Legality
- In the United States, 5F-APINACA is classified as a Schedule I controlled substance.
- Germany has designated 5F-APINACA as an Anlage II controlled drug since July 2013.
- As of October 2015, 5F-APINACA is categorized as a controlled substance in China.
- The Czech Republic has implemented a ban on 5F-APINACA.
FAQ
1. What is 5F-APINACA?
5F-APINACA is a synthetic cannabinoid known for interacting with cannabinoid receptors in the body, specifically CB1 and CB2 receptors.
2. What is the binding affinity of 5F-APINACA at CB1 and CB2 cannabinoid receptors?
5F-APINACA exhibits a high binding affinity of 1.94 nM at CB1 and 0.266 nM at CB2 receptors, making it a potent agonist.
3. Is 5F-APINACA legal in the United States?
No, 5F-APINACA is classified as a Schedule I controlled substance in the United States, making it illegal to possess, distribute, or use.
4. What is the legal status of 5F-APINACA in Germany?
In Germany, 5F-APINACA is categorized as an Anlage II controlled drug, a classification that has been in place since July 2013.
5. Is 5F-APINACA controlled in China?
Yes, as of October 2015, 5F-APINACA is considered a controlled substance in China.
6. Has 5F-APINACA been banned in any other countries?
Yes, 5F-APINACA is banned in the Czech Republic.
7. Are there any documented health risks associated with 5F-APINACA use?
The use of synthetic cannabinoids like 5F-APINACA has been associated with various health risks, including adverse effects on physical and mental health. Users should be aware of these risks and avoid its consumption.
8. Where can I find more information about 5F-APINACA and its effects?
For more information about 5F-APINACA and its potential effects, consult credible sources such as government agencies, healthcare professionals, and substance abuse organizations. Staying informed is crucial for making safe choices regarding substance use.
References
- Anvisa (2023-07-24). “RDC Nº 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial” [Collegiate Board Resolution No. 804 – Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- Explore “AKB48 N-(5-fluoropentyl) analog” at Cayman Chemical, retrieved on 6 July 2015.
- Access the detailed information on “5F-AKB48” in the PDF document from the Scientific Working Group for the Analysis of Seized Drugs (SWGDRUG), dated 18 February 2013, retrieved on 6 July 2015.
- Learn more about the relevant patent WO 2003/035005.
- Chung H, Choi H, Heo S, Kim E, Lee J (January 2014). “Synthetic cannabinoids abused in South Korea: drug identifications by the National Forensic Service from 2009 to June 2013”. Forensic Toxicology. 32 (1): 82–88. doi:10.1007/s11419-013-0213-6. S2CID 23058813.
- Gain insights from the Drug Enforcement Administration’s document on “AKB48 (APINACA) and 5F-AKB48 (5F-APINACA),” dated May 2013.
- Jang M, Shin I, Kim J, Yang W (February 2015). “Simultaneous quantification of 37 synthetic cannabinoid metabolites in human urine by liquid chromatography-tandem mass spectrometry”. Forensic Toxicology. 33 (2): 221–234. doi:10.1007/s11419-015-0265-x. S2CID 3038555.
- Karinen R, Tuv SS, Øiestad EL, Vindenes V (January 2015). “Concentrations of APINACA, 5F-APINACA, UR-144 and its degradant product in blood samples from six impaired drivers compared to previous reported concentrations of other synthetic cannabinoids”. Forensic Science International. 246: 98–103. doi:10.1016/j.forsciint.2014.11.012. PMID 25485949.
- Holm NB, Pedersen AJ, Dalsgaard PW, Linnet K (March 2015). “Metabolites of 5F-AKB-48, a synthetic cannabinoid receptor agonist, identified in human urine and liver microsomal preparations using liquid chromatography high-resolution mass spectrometry”. Drug Testing and Analysis. 7 (3): 199–206. doi:10.1002/dta.1663. PMID 24802286.
- Wohlfarth A, Castaneto MS, Zhu M, Pang S, Scheidweiler KB, Kronstrand R, Huestis MA (May 2015). “Pentylindole/Pentylindazole Synthetic Cannabinoids and Their 5-Fluoro Analogs Produce Different Primary Metabolites: Metabolite Profiling for AB-PINACA and 5F-AB-PINACA”. The AAPS Journal. 17 (3): 660–77. doi:10.1208/s12248-015-9721-0. PMC 4406957. PMID 25721194.
- Hess C, Schoeder CT, Pillaiyar T, Madea B, Müller CE (1 July 2016). “Pharmacological evaluation of synthetic cannabinoids identified as constituents of spice”. Forensic Toxicology. 34 (2): 329–343. doi:10.1007/s11419-016-0320-2. PMC 4929166. PMID 27429655.
- “Schedules of Controlled Substances: Temporary Placement of Six Synthetic Cannabinoids (5F-ADB, 5F-AMB, 5F-APINACA, ADB-FUBINACA, MDMB-CHMICA and MDMB-FUBINACA) Into Schedule I.” Document from the Drug Enforcement Administration. Archived from the original on 2019-10-17. Retrieved on 2017-03-17.
- “关于印发《非药用类麻醉药品和精神药品列管办法》” (in Chinese). Notice from the China Food and Drug Administration, dated 27 September 2015. Archived from the original on 1 October 2015.
- “Látky, o které byl doplněn seznam č. 4 psychotropních látek (příloha č. 4 k nařízení vlády č. 463/2013 Sb.)” (PDF) (in Czech). Ministerstvo zdravotnictví. Archived from the original (PDF) on 2016-03-09. Retrieved on 2016-02-06.