Summary
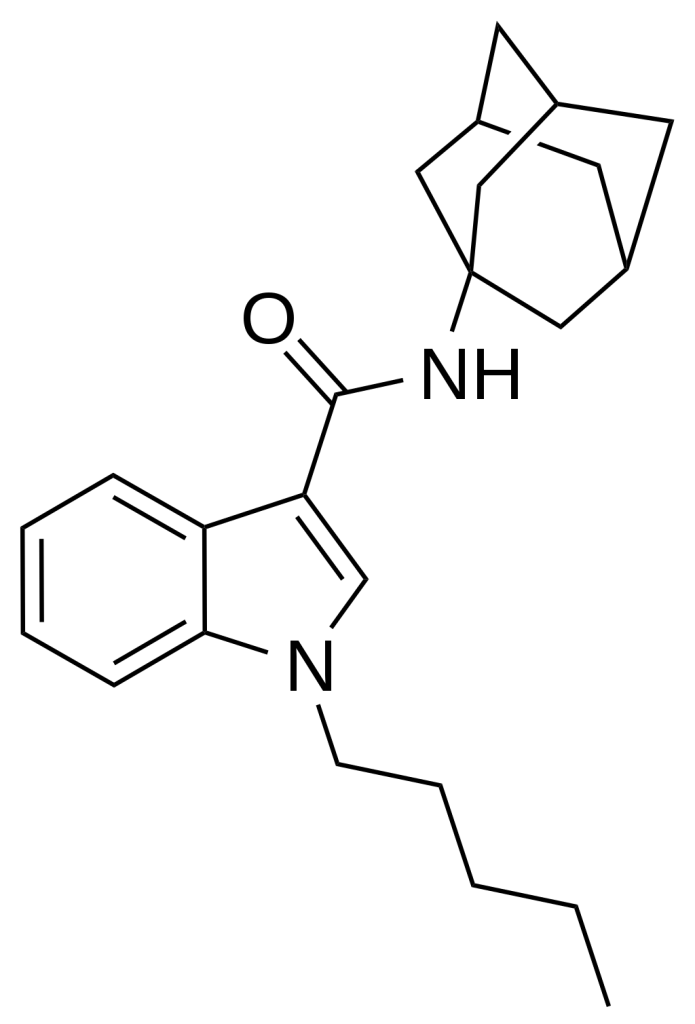
APICA also referred to as 2NE1, SDB-001, or N-(1-adamantyl)-1-pentyl-1H-indole-3-carboxamide, stands as an indole-based compound renowned for its robust agonistic effects on cannabinoid receptors.
This distinctive compound debuted in the scientific and patent literature when laboratories in Japan identified it in March 2012. It was found as an ingredient in synthetic cannabis smoking blends, notably in conjunction with its indazole derivative, APINACA, which was marketed as “AKB48.”
Structurally, APICA closely resembles certain cannabinoid compounds featured in patent WO 2003/035005. However, it diverges by possessing an indole core instead of indazole alongside a straightforward pentyl chain attached to the indole 1-position. Notably, given the presence of amantadine as a metabolic byproduct in the related compound APINACA, there is a suspicion that metabolic hydrolysis of the amide group in APICA may also liberate amantadine.
Pharmacological evaluations have revealed that APICA exhibits a significant IC50 value of 175 nM at the CB1 receptor, just slightly less potent than JWH-018, which boasted an IC50 of 169 nM. Compared to APINACA, with an IC50 of 824 nM, APICA binds over four times more tightly to the CB1 receptor.
Moreover, the first published synthesis and pharmacological assessment of APICA unveiled its status as a full agonist at both CB1 (EC50 = 34 nM) and CB2 receptors (EC50 = 29 nM). Notably, APICA has been found to induce cannabis-like effects in rats, positioning it as less potent than JWH-018 but more potent than THC in terms of its physiological effects.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1345973-50-3 |
|---|---|
| PubChem CID | 71308155 |
| ChemSpider | 29341717 |
| UNII | HKU510FH74 |
| CompTox Dashboard (EPA) | DTXSID80745427 |
| Chemical and physical data | |
| Formula | C24H32N2O |
| Molar mass | 364.533 g·mol−1 |
Legal Status
As of October 2015 APICA is a controlled substance in China.
FAQ
1. What is APICA?
APICA is a synthetic cannabinoid drug that acts as a potent agonist for cannabinoid receptors. It is known by several names, including 2NE1, SDB-001, and N-(1-adamantyl)-1-pentyl-1H-indole-3-carboxamide.
2. How was APICA discovered?
APICA had not been previously reported in scientific or patent literature before its identification in March 2012. It was first identified by laboratories in Japan as an ingredient in synthetic cannabis smoking blends, often alongside the indazole derivative APINACA (marketed as “AKB48”).
3. What is the chemical structure of APICA?
APICA is structurally distinguished by its indole core instead of indazole and features a simple pentyl chain on the indole 1-position.
4. Are there concerns about the release of amantadine through metabolic processes in APICA?
Yes, there is a suspicion that metabolic hydrolysis of the amide group in APICA may release amantadine, given its presence as an impurity in the related compound APINACA.
5. How potent is APICA at cannabinoid receptors?
APICA exhibits a notable IC50 value of 175 nM at the CB1 receptor, making it slightly less potent than the well-known JWH-018, which has an IC50 of 169 nM. Compared to APINACA, APICA binds over four times more tightly to the CB1 receptor.
6. What are the pharmacological effects of APICA?
APICA is a full agonist at the CB1 (EC50 = 34 nM) and CB2 receptors (EC50 = 29 nM). It has been found to induce cannabis-like effects in animal studies and appears to be less potent than JWH-018 but more potent than THC in terms of its physiological effects.
7. Is APICA legal and safe for use?
The legal status of APICA varies by region and country, and its safety is a subject of concern. The use of synthetic cannabinoids can have unpredictable and potentially harmful effects. Users should be aware of and adhere to local regulations.
8. Can APICA be used for medical purposes?
APICA is primarily recognized as a research chemical and not for medical use. Its potential therapeutic applications still need to be established.
9. What are the potential risks associated with APICA use?
Using synthetic cannabinoids like APICA can pose various risks, including potential health hazards, unknown long-term effects, and possible legal consequences.
10. Where can I find more information about APICA?
To gain a more comprehensive understanding of APICA, consider referring to scientific literature, research studies, and relevant authorities. Staying informed about the latest developments is crucial.
References
- In August 2016, Cannizzaro C, Malta G, Argo A, and their team conducted an extensive study, providing a “Behavioural and pharmacological characterization of a novel cannabinomimetic adamantane-derived indole, APICA.” This research delved into the potential misuse of APICA as a psychotropic substance, particularly in C57bl/6J mice. The findings were documented in “Forensic Science International,” Volume 265, spanning pages 6–12. To explore further details, you can refer to doi:10.1016/j.forsciint.2015.12.035. The paper is also accessible at hdl:2434/422068, and the PMID for this publication is 26826846.
- In 2012, Uchiyama N, Kawamura M, and Kikura-Hanajiri R, among others, made significant strides in the identification of two new-type synthetic cannabinoids, APICA and APINACA, and the detection of five synthetic cannabinoids, such as AM-1220, AM-2233, AM-1241, CB-13 (CRA-13), and AM-1248. Their research focused on designer drugs found in illegal products and was published in “Forensic Toxicology,” Volume 30, Issue 2, covering pages 114–125. To delve further into their findings, you can refer to doi:10.1007/s11419-012-0136-7. The paper is also available on S2CID 44193953.
- In April 2013, Uchiyama N, Kawamura M, and Kikura-Hanajiri R conducted research on “URB-754,” a new class of designer drug, and identified 12 synthetic cannabinoids detected in illegal products. This study was published in “Forensic Science International,” Volume 227, covering pages 21–32. For more details, you can refer to doi:10.1016/j.forsciint.2012.08.047, and the PMID for this publication is 23063179.
- In July 2013, Banister SD, Wilkinson SM, and their research team synthesized and pharmacologically evaluated adamantane-derived indoles, particularly focusing on cannabimimetic drugs of abuse. This work was published in “ACS Chemical Neuroscience,” Volume 4, Issue 7, spanning pages 1081–1092. To explore further insights, refer to doi:10.1021/cn400035r. Additionally, this publication is accessible on PMC (PubMed Central) with the identifier PMC 3715837, and the PMID is 23551277.
- In August 2015, Banister SD, Stuart J, Kevin RC, and their colleagues investigated the effects of bioisosteric fluorine in various synthetic cannabinoid designer drugs. This study covered compounds such as JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135. Their findings were published in “ACS Chemical Neuroscience,” Volume 6, Issue 8, with the article spanning pages 1445–1458. For more information, refer to doi:10.1021/acschemneuro.5b00107, and the PMID for this publication is 25921407.
- The “关于印发《非药用类麻醉药品和精神药品列管办法》的通知” (in Chinese) was issued by the China Food and Drug Administration on September 27, 2015. It pertains to the administration’s notification regarding the “Measures for the Supervision and Control of Non-Medicinal Narcotic Drugs and Psychotropic Drugs.” This notification is a significant regulatory development in the control of such substances.