Summary
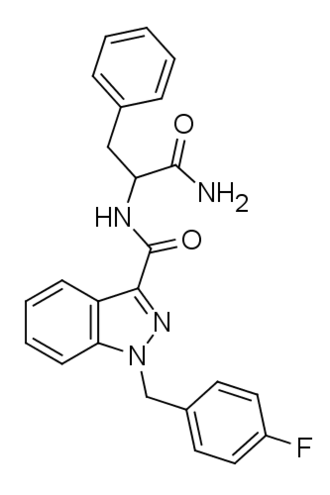
APP-FUBINACA is a synthetic cannabinoid based on the indazole structure, and it has been distributed online as a designer drug. Regarding its pharmacological profile, research has indicated that APP-FUBINACA exhibits only moderate binding affinity for the CB1 receptor, with a Ki value of 708 nM. However, specific data regarding its EC50 has yet to be evaluated. Notably, this compound features a chemical structure with a phenylalanine amino acid residue.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 1185282-03-4 |
|---|---|
| PubChem CID | 58124515 |
| UNII | TW71LSK9DG |
| Chemical and physical data | |
| Formula | C24H21FN4O2 |
| Molar mass | 416.456 g·mol−1 |
Legality
On March 24, 2015, Sweden’s public health agency proposed the classification of APP-FUBINACA as a hazardous substance.
FAQ
- What is APP-FUBINACA?
- APP-FUBINACA is an indazole-based synthetic cannabinoid that has been sold online as a designer drug.
- How does APP-FUBINACA affect the body?
- APP-FUBINACA has a moderate affinity for the CB1 receptor, a component of the endocannabinoid system. It may produce effects like other cannabinoids, such as altered perception, relaxation, and mood changes.
- Is APP-FUBINACA legal?
- Legal status varies by country. It is important to check your local laws and regulations regarding the possession, sale, or use of APP-FUBINACA. In many places, it is classified as a controlled substance due to its potential health risks.
- What are the potential risks associated with APP-FUBINACA?
- The use of synthetic cannabinoids like APP-FUBINACA can be associated with various health risks, including cardiovascular problems, respiratory issues, and mental health effects. It is crucial to be aware of the potential dangers and use such substances responsibly.
- Why did Sweden’s public health agency propose classifying APP-FUBINACA as a hazardous substance?
- Public health agencies often make recommendations based on scientific evidence and concerns about the safety of certain substances. The proposal to classify APP-FUBINACA as a hazardous substance was likely made to address potential health risks associated with its use.
- Is there any medical or therapeutic use for APP-FUBINACA?
- Synthetic cannabinoids like APP-FUBINACA are not approved for medical use and are not considered suitable for therapeutic purposes. These substances are primarily produced and used for recreational purposes, which may pose health risks.
- Where can I find more information about APP-FUBINACA and its legal status?
- It’s essential to consult your local drug enforcement agency or health department for up-to-date information on the legal status of APP-FUBINACA in your region. Staying informed about local laws and regulations is crucial to ensure compliance with the law.
References
- APP-FUBINACA: An Overview APP-FUBINACA is an indazole-based synthetic cannabinoid that gained notoriety as a designer drug. This compound has drawn attention due to its potential effects on the endocannabinoid system.
- Origin and Development Originally, this synthetic cannabinoid was mentioned in a patent from Pfizer Inc. dated September 3, 2009. The patent detailed “Indazole derivatives,” which included APP-FUBINACA as a part of this chemical family.
- Legal Classification in Sweden Sweden’s public health agency has proposed classifying APP-FUBINACA as a hazardous substance. Such recommendations are made based on scientific evidence and concerns about the safety of the substance.
- Pharmacological Testing APP-FUBINACA exhibits only moderate affinity for the CB1 receptor. The Ki (binding affinity) for this receptor is measured at 708 nM, while the EC50 (effectiveness) was not tested. This moderate affinity for the CB1 receptor suggests that its effects may differ from other synthetic cannabinoids with higher binding affinity.
- Recent Research In recent years, APP-FUBINACA has been the subject of in vitro research to evaluate its activity on cannabinoid receptors. This research has examined its interaction with the CB1 receptor and provided insights into its pharmacological effects.