Contents
Summary
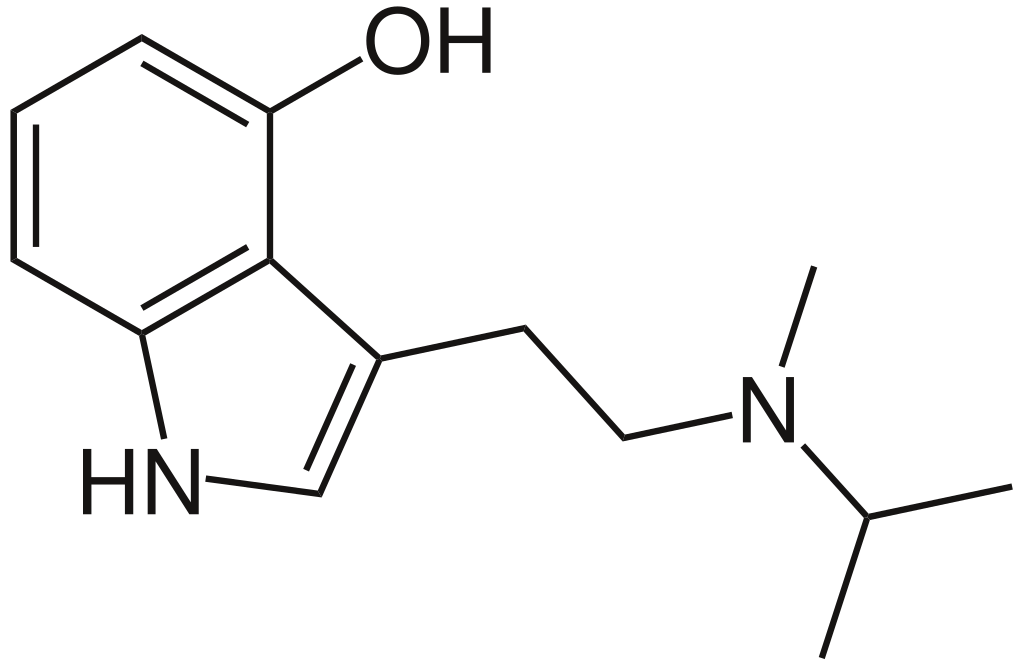
4-HO-MiPT, also known as miprocin or 4-hydroxy-N-methyl-N-isopropyltryptamine, is a synthetic aromatic compound that falls into the category of lesser-known psychedelic tryptamines. This substance is believed to possess serotonergic psychedelic properties, making it comparable to psychedelics like magic mushrooms, LSD, and mescaline. Its molecular structure and pharmacological effects bear some resemblance to those of psilocin, the primary psychoactive compound found in magic mushrooms.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 77872-43-6 |
|---|---|
| PubChem CID | 10082683 |
| ChemSpider | 8258221 |
| UNII | 4GAJ9OJ8YZ |
| ChEMBL | ChEMBL171419 |
| CompTox Dashboard (EPA) | DTXSID30228492 |
| Chemical and physical data | |
| Formula | C14H20N2O |
| Molar mass | 232.327 g·mol−1 |
History
4-HO-MiPT is believed to have been initially synthesized by Alexander Shulgin. Details of its synthesis can be found in his book “TiHKAL,” which also includes reports from individuals who have consumed this compound. Shulgin’s experimental trials and additional anecdotal accounts collectively indicate that 4-HO-MiPT exhibits synthetic psychedelic effects akin to psilocin. It’s worth noting that this substance is relatively rare and has a limited history of human usage.
Chemistry
Miprocin is the 4-hydroxyl analog of N-methyl-N-isopropyltryptamine and serves as the isopropyl homolog, potentially sharing structural similarities with 4-HO-DMT.
In August 2019, Chadeayne et al. determined the crystal structure of 4-HO-MiPT fumarate. This compound’s systematic name is 2-(4-hydroxy-1H-indol-3-yl)ethylpropan-2-ylazanium 3-carboxyprop-2-enoate monohydrate. The fumarate salt consists of a protonated tryptammonium cation and a 3-carboxy acrylate (hydrogen fumarate) anion in the asymmetric unit, accompanied by a water molecule necessary for crystallization.
Pharmacology
4-HO-MiPT is considered to be a serotonergic psychedelic compound. Similar to other substances in this category, its mode of action is believed to stem from its partial agonism of serotonin receptors, particularly 5-HT2A and 5-HT1A receptors.
Toxicity
The toxicity profile of 4-HO-MiPT remains largely unknown. While its chemical structure and pharmacological properties bear similarities to psilocin, a substance not typically associated with compulsive use or physical dependence, the limited research on 4-HO-MiPT prevents definitive conclusions regarding its actions in the human body compared to psilocin. Notably, there have been no reported fatalities attributed to 4-HO-MiPT use to date.
Drug prohibition laws
Sweden:
In Sweden, the health ministry of Sveriges riksdag, Statens folkhälsoinstitut, categorized 4-HO-MiPT as a “health hazard” under the Act on the Prohibition of Certain Goods Dangerous to Health, effective from November 1, 2005. It is listed as “4-hydroxy-N, N-metylisopropyltryptamin (4-HO-MIPT),” making its sale and possession illegal.
United Kingdom:
4-HO-MiPT may be considered illegal in the United Kingdom under the Misuse of Drugs Act 1971.
United States:
4-HO-MiPT is currently an unscheduled substance in the United States. However, it could be treated as an analog of psilocin, potentially leading to legal prosecution under the Federal Analog Act.
Russia:
Russia classifies 4-HO-MiPT under Schedule 1 as an analog of 4-hydroxytryptamine.
FAQ
- What is 4-HO-MiPT?
- 4-HO-MiPT, or miprocin, is a synthetic psychedelic compound in the tryptamine class. It shares structural similarities with psilocin, the primary psychoactive component found in magic mushrooms, and is believed to produce serotonergic psychedelic effects.
- Who synthesized 4-HO-MiPT?
- 4-HO-MiPT was presumably first synthesized by Alexander Shulgin, who documented its synthesis and effects in his book “TiHKAL” (Tryptamines I Have Known and Loved).
- What are the effects of 4-HO-MiPT?
- Users of 4-HO-MiPT report effects similar to those of psilocin, including mydriasis (dilated pupils), closed and open eye visuals, euphoria, time dilation, and alterations in thought processes. The effects typically occur in waves, similar to psilocybin, with rapid variations in perception.
- What is the typical duration of 4-HO-MiPT effects?
- The effects of 4-HO-MiPT typically last for approximately 4 to 6 hours when administered orally and 3 to 4 hours when taken intranasally.
- Is 4-HO-MiPT toxic or associated with physical dependence?
- Very little is known about the toxicity of 4-HO-MiPT. Its chemical structure and pharmacological activity are similar to psilocin, a compound not commonly linked to compulsive use or physical dependence. However, more research is needed to assess its pharmacological actions and long-term effects definitively.
- Has there been any reported deaths associated with 4-HO-MiPT use?
- To date, there have been no reported fatalities attributed to the use of 4-HO-MiPT.
- Is 4-HO-MiPT legal in different countries?
- The legal status of 4-HO-MiPT varies by country. For example, it is classified as a “health hazard” and illegal to sell or possess in Sweden. In the United Kingdom, it could be considered unfair under the Misuse of Drugs Act 1971. In the United States, it is unscheduled but could potentially be prosecuted under the Federal Analog Act. In Russia, it is in Schedule 1 as an analog of 4-hydroxytryptamine.
References
- Anvisa (24 July 2023). “Resolution No. 804 of the Collegiate Board – Compilations of Controlled Narcotic, Psychotropic, Precursor, and Other Special Substances” [Resolução da Diretoria Colegiada No. 804 – Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial] (in Brazilian Portuguese). Published in Diário Oficial da União on 25 July 2023. Archived from the original source on 27 August 2023. Retrieved on 27 August 2023.
- “4-HO-MiPT”. Psychedelic Science Review. Published on 6 January 2020. Retrieved on 5 July 2022.
- Chadeayne AR, Pham DN, Golen JA, Manke DR (September 2019). “N-methyl Derivatives of DMT and Psilocin”. Acta Crystallographica Section E. Volume 75, Part 9, Pages 1316–1320. doi:10.1107/S2056989019011253. PMC 6727059. PMID 31523457.
- “Regulation Amending the Ordinance (1999:58) on Prohibition Against Certain Dangerous Goods” [Förordning om ändring i förordningen (1999:58) om förbud mot vissa hälsofarliga varor]. Published in Swedish Code of Statutes (in Swedish) on 6 October 2005. Archived from the original (PDF) on 26 June 2021. Retrieved on 6 September 2013.