Isopropylphenidate, often abbreviated as IPPH, is a synthetic stimulant that has recently gained popularity, primarily among the research chemical community. As with many research chemicals, the accessibility of IPPH has led to its availability through various online vendors. This review critically examines the landscape of IPPH research chemical sellers, shedding light on the risks and considerations associated with buying this designer drug online.
First and foremost, it is essential to acknowledge the proliferation of online vendors offering IPPH for sale. While the internet provides a platform for convenient access to various research chemicals, it also presents substantial challenges concerning these substances’ quality, safety, and legality. Many sellers operate without proper regulation or oversight, making it difficult for consumers to trust the source of their research chemicals.
One significant concern is the inconsistency in the purity and composition of IPPH sold by different vendors. Researchers seeking accurate and reliable results must contend with the uncertainty surrounding the chemical’s integrity. The lack of standardized production and quality control measures within the research chemical industry further exacerbates this issue.
Moreover, the legality of IPPH varies from jurisdiction to jurisdiction, adding complexity to the decision to buy from online vendors. Researchers must be well-informed about the regulations in their region to avoid legal consequences. The classification of research chemicals can change, and vendors and buyers must stay updated on these developments.
Contents
- 1 Summary
- 2 Chemistry
- 3 Pharmacology
- 4 Subjective effects
- 5 Toxicity
- 6 Dosage
- 7 Legal status
- 7.1 FAQ
- 7.2 1. What is Isopropylphenidate?
- 7.3 2. What are the effects of Isopropylphenidate?
- 7.4 3. Is Isopropylphenidate legal?
- 7.5 4. What are the risks of using Isopropylphenidate?
- 7.6 5. How is Isopropylphenidate used?
- 7.7 6. Is Isopropylphenidate safe?
- 7.8 7. Can Isopropylphenidate cause addiction?
- 7.9 8. Are there any known dangerous drug interactions with Isopropylphenidate?
- 7.10 9. Can I use Isopropylphenidate for medical purposes?
- 8 References
Summary
Isopropylphenidate, known by various abbreviations such as IPH, IPPH, IPD, and IPPD, belongs to the lesser-known stimulant category within the phenidate chemical class. This compound resembles the widely prescribed medication for ADHD, methylphenidate, and serves as a norepinephrine-dopamine reuptake inhibitor (NDRI).
Researchers have explored the potential of Isopropylphenidate as an alternative to methylphenidate in managing ADHD and related conditions. Studies indicate that it exhibits similar foundational characteristics to a norepinephrine-dopamine reuptake inhibitor (NDRI). It demonstrates a notable affinity for the dopamine transporter and influences its cellular reuptake, akin to methylphenidate and methylphenidate. Notably, its impact on norepinephrine appears modest, suggesting a potentially more favourable safety and toxicity profile.
Subjective effects associated with Isopropylphenidate encompass stimulation, heightened motivation, appetite suppression, and euphoria. Its cognitive and physical outcomes are often likened to methylphenidate, although with a milder euphoric component and an extended duration of action. These characteristics have led some to propose its suitability as a study aid or productivity enhancer.
Isopropylphenidate has only a brief history of recreational use among humans. Its emergence followed the prohibition of methylphenidate, which became illegal in the United Kingdom in April 2015, initially under a temporary ban that later became permanent. Subsequently, Isopropylphenidate became accessible through online grey-market channels as a research chemical intended for global distribution. As of 2022, Isopropylphenidate continues to be obtainable and exists in a legally ambiguous status across many regions worldwide, primarily distributed by online research chemical vendors.
| Identifiers | |
|---|---|
| showIUPAC name | |
| CAS Number | 93148-46-0 |
| PubChem CID | 68314762 |
| ChemSpider | 48062090 |
| UNII | N93Y7VG3XF |
| Chemical and physical data | |
| Formula | C16H23NO2 |
| Molar mass | 261.365 g·mol−1 |
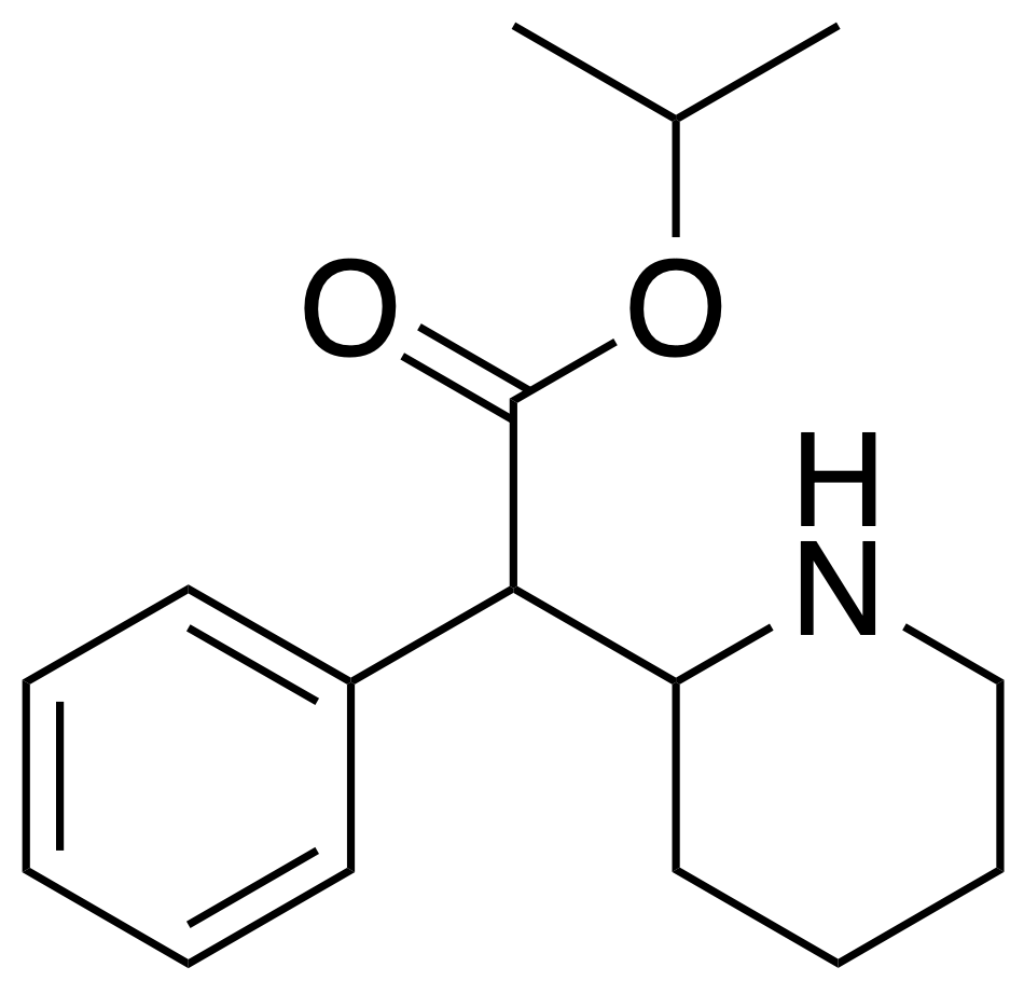
Chemistry
Isopropylphenidate belongs to synthetic molecules, specifically categorized within the substituted phenethylamine, amphetamines, and phenidate classes. Its fundamental structure centres around a phenethylamine core, characterized by a phenyl ring connected to an amino (NH2) group through an ethyl chain. Notably, it shares structural similarities with amphetamine, encompassing a substitution at Rα, leading to the formation of a piperidine ring that culminates in the terminal amine of the phenethylamine chain. Moreover, Isopropylphenidate features an isopropyl acetate group attached to Rβ, marking a distinct deviation from methylphenidate, where this position typically contains a methyl group.
The structural distinction between Isopropylphenidate, ethylphenidate, and methylphenidate lies in the carbon chain length within their respective acetate groups. The prefix “Iso-” references the side chain with one carbon atom branching into two methyl groups, while “Phen-” alludes to the phenyl ring. “Id-” is a contraction denoting the presence of the piperidine ring, and “-ate” designates the acetate group. It’s worth noting that Isopropylphenidate exists in a chiral form, meaning it can be found as a racemic mixture containing both enantiomers or exclusively as one of its enantiomers.
Pharmacology
Formal in-vivo human trials investigating the effects of isopropylphenidate are currently lacking. However, rat studies conducted in vivo have demonstrated stimulatory effects, while in vitro research has focused on assessing binding affinities to monoamine transporters and various hydrolytic enzymes.
The findings from these studies indicate that isopropylphenidate shares a very similar pharmacological profile with its parent compound, methylphenidate. Notable distinctions between the two substances include isopropylphenidate’s significantly reduced activity as a norepinephrine reuptake inhibitor and its interaction with the CES1 hydrolytic enzyme. This enzyme breaks down both compounds into ritalinic acid, with isopropylphenidate exhibiting an 8-fold lower affinity for CES1 than methylphenidate.
These disparities translate into distinct effects when comparing isopropylphenidate to methylphenidate. Isopropylphenidate displays more pronounced dopaminergic activity than adrenergic at equivalent effective doses. Furthermore, it boasts a longer duration of action and greater potency than methylphenidate at equivalent dosages. Notably, the heightened potency of isopropylphenidate, especially when administered orally, is attributed to the lower CES1 affinity, which increases its bioavailability compared to methylphenidate. This increased bioavailability is particularly significant because methylphenidate’s oral administration results in notably low bioavailability due to extensive first-pass metabolism in the liver mediated by CES1.
Despite these notable differences, isopropylphenidate is primarily believed to function as both a dopamine reuptake inhibitor and a norepinephrine reuptake inhibitor. In practical terms, isopropylphenidate effectively elevates dopamine and norepinephrine neurotransmitters in the brain by binding to and partially inhibiting the transporter proteins responsible for removing these monoamines from the synaptic cleft. Consequently, this accumulation of dopamine and norepinephrine leads to stimulatory effects.

Subjective effects
Disclaimer: The following effects are based on the Subjective Effect Index (SEI), a compilation of anecdotal user reports and analyses by contributors to PsychonautWiki. These effects should be approached with a healthy degree of scepticism.
It’s important to note that these effects may not manifest predictably or reliably. However, higher doses are more likely to produce the full range of effects. Adverse effects become more likely with increased doses, including addiction, severe injury, or even death ☠.
Physical:
- Stimulation: Isopropylphenidate is typically described as providing energy and stimulation, albeit to a lesser extent than amphetamine or methamphetamine and stronger than modafinil and caffeine. Lower to moderate doses promote general productivity, while higher doses may encourage physical activities like dancing, socializing, running, or cleaning. This stimulation can become forced at higher doses, leading to jaw clenching, bodily shakes, and vibrations, resulting in loss of motor control.
- Dehydration
- Dry Mouth
- Appetite Suppression
- Vasoconstriction
- Increased Heart Rate
- Mouth Numbing: Sublingual administration can lead to a long-lasting numbing sensation.
- Teeth Grinding: Although less intense compared to MDMA.
After:
- During the offset of a stimulant experience, the comedown phase is generally uncomfortable and negative, attributed to neurotransmitter depletion. Effects may include:
- Anxiety
- Cognitive Fatigue
- Depression
- Irritability
- Motivation Suppression
- Thought Deceleration
- Wakefulness
Cognitive:
- Isopropylphenidate’s cognitive effects intensify progressively with dosage. It is often associated with mental stimulation, increased focus, and manageable euphoria, including typical stimulant cognitive effects. Negative side effects tend to be mild at low to moderate doses but more likely with higher or prolonged use. Prominent cognitive effects include:
- Analysis Enhancement
- Focus Enhancement: Most effective at low to moderate doses; higher doses may impair concentration.
- Motivation Enhancement
- Stamina Enhancement
- Thought Acceleration
- Thought Connectivity
- Cognitive Euphoria: A short-lived and compulsive euphoric rush similar to cocaine, resulting from dopamine reuptake inhibition.
- Increased Music Appreciation
- Increased Libido: Generally milder compared to other stimulants.
- Irritability: More noticeable at higher doses, especially during the peak and offset, possibly due to its high dopaminergic activity relative to noradrenergic activity.
- Time Distortion: The perception of time speeding up and passing quickly.
- Compulsive Redosing: This urge is often associated with the mild-to-moderate euphoric rush and the short half-life of isopropylphenidate, especially when insufflated. Users looking to use it for productivity are advised to stick to low to moderate oral doses to avoid compulsive redosing, which tends to be less pronounced than other research chemical stimulants.
- Wakefulness
Please remember that individual reactions to substances can vary, and the effects mentioned above are based on anecdotal reports. Exercise caution and responsible use when considering isopropylphenidate or any other substance.
Toxicity
The toxicity and potential long-term health consequences of recreational isopropylphenidate use have not been systematically studied within the scientific community. Consequently, the precise toxic dosage remains unknown due to isopropylphenidate’s limited history of human consumption. Anecdotal reports from individuals who have experimented with isopropylphenidate within the community suggest that there have been no apparent adverse health effects when used in moderation at low to moderate doses. However, it is essential to exercise caution, recognizing that no guarantees regarding safety exist. Therefore, it is strongly advisable to incorporate harm-reduction practices when considering the use of this substance.
Tolerance and Addiction Potential:
Like other stimulants, chronic isopropylphenidate use can be moderately addictive and carries a high potential for abuse, potentially leading to psychological dependence in certain users. Those who develop an addiction may experience cravings and withdrawal symptoms if they abruptly cease usage.
Tolerance to isopropylphenidate’s effects tend to develop with prolonged and repeated use, necessitating higher doses to achieve the same effects. It takes approximately 3 to 7 days for the tolerance to decrease by half and 1 to 2 weeks to return to baseline (without further consumption). It’s important to note that isopropylphenidate induces cross-tolerance with all dopaminergic stimulants, meaning that after its consumption, the effects of other stimulants are reduced.
Psychosis:
Prolonged and high-dosage use of stimulants, including isopropylphenidate, can potentially lead to stimulant-induced psychosis, characterized by symptoms like paranoia, hallucinations, and delusions. Recovery from such psychosis can be challenging, with some users failing to recover fully. Antipsychotic medications have been reported to alleviate acute amphetamine psychosis effectively.
Dangerous Interactions:
It is crucial to be aware that combining psychoactive substances, even those considered safe individually, can become hazardous or life-threatening when combined with specific other substances. Below is a list of known dangerous interactions (although it may not encompass all possibilities):
- 25x-NBOMe & 25x-NBOH: These compounds are highly stimulating and physically demanding. Combining them with Isopropylphenidate should be strictly avoided due to the risk of excessive stimulation, heart strain, increased blood pressure, panic attacks, thought loops, seizures, and potential heart failure in extreme cases.
- Alcohol: Mixing alcohol with stimulants can be dangerous as stimulants mask the depressant effects of alcohol. Once the stimulant’s effects wear off, the depressant effects can predominate, leading to blackouts and severe respiratory depression. If combining these substances, it’s crucial to limit alcohol intake per hour.
- DXM: Combining DXM with Isopropylphenidate is discouraged due to DXM’s inhibitory effects on serotonin and norepinephrine reuptake, increasing the risk of panic attacks, hypertensive crisis, or serotonin syndrome when combined with serotonin releasers like MDMA, methylone, and mephedrone.
- MDMA: The neurotoxic effects of MDMA may be heightened when combined with other stimulants. Additionally, there is a risk of elevated blood pressure and heart strain (cardiotoxicity).
- MXE: Some reports suggest that combining MXE with Isopropylphenidate may dangerously elevate blood pressure and increase the risk of mania and psychosis.
- Dissociatives: Both substances risk delusions, mania, and psychosis, which may be exacerbated when used together.
- Stimulants: Combining Isopropylphenidate with other stimulants like cocaine can potentially increase heart rate and blood pressure to dangerous levels.
- Tramadol: Tramadol is known to lower the seizure threshold, and combining it with stimulants may further increase this risk.
- MAOIs: Combining Isopropylphenidate with MAOIs may lead to dangerously high levels of neurotransmitters such as dopamine, which can be fatal. Examples of MAOIs include Syrian rue, banisteriopsis caapi, and certain antidepressants.
Dosage
| Dosage | |
|---|---|
| Threshold | 2 mg |
| Light | 5 – 15 mg |
| Common | 15 – 25 mg |
| Strong | 25 – 45 mg |
| Heavy | 45 mg + |
| Duration | |
| Total | 3.5 – 6 hours |
| Onset | 10 – 30 minutes |
| Come up | 20 – 40 minutes |
| Peak | 1.5 – 2.5 hours |
| Offset | 1 – 2 hours |
Legal status
Germany: Isopropylphenidate was classified as a controlled substance under the NpSG (New Psychoactive Substances Act) on November 26, 2016. This legislation makes the production and import of isopropylphenidate with the intent to market, its administration to others, and trading punishable by law. While possession is illegal, it is not subject to penalization.
Switzerland: Isopropylphenidate is designated as a controlled substance and is explicitly listed under Verzeichnis E.
Turkey: Isopropylphenidate has been illegal in Turkey since February 2016.
United Kingdom: As of May 31, 2017, Isopropylphenidate is classified as a class B drug in the United Kingdom. Consequently, possessing, producing, or supplying this substance is illegal.
Canada: Isopropylphenidate is categorized as a Schedule III drug in Canada, subject to regulations governing its control.
FAQ
1. What is Isopropylphenidate?
Isopropylphenidate, or IPH or IPPH, is a synthetic stimulant belonging to the phenidate class. It is structurally similar to methylphenidate, a medication for attention deficit hyperactivity disorder (ADHD). Isopropylphenidate is known for its stimulating effects on the central nervous system.
2. What are the effects of Isopropylphenidate?
The effects of Isopropylphenidate can include increased alertness, focus, and motivation. It may also lead to physical effects like stimulation, decreased appetite, and increased heart rate. Users have reported mood enhancement and a sense of euphoria, although this can vary between individuals.
3. Is Isopropylphenidate legal?
The legal status of Isopropylphenidate varies by country. In some places, it is classified as a controlled substance and is illegal to possess, produce, or distribute. In other regions, it may fall into a legal grey area. It’s essential to research the specific regulations in your area before considering its use.
4. What are the risks of using Isopropylphenidate?
Using Isopropylphenidate comes with potential risks. It can be addictive, and chronic use may lead to tolerance, dependence, and withdrawal symptoms. It can also cause adverse physical and psychological effects, such as anxiety, irritability, and insomnia. Combining Isopropylphenidate with other substances, including alcohol, can be dangerous and increase health risks.
5. How is Isopropylphenidate used?
Isopropylphenidate is typically taken orally but can also be insufflated (snorted). The dosage varies depending on individual tolerance and the desired effects. It’s crucial to start with a low dose and exercise caution to avoid potential health complications.
6. Is Isopropylphenidate safe?
The safety of Isopropylphenidate is not well-studied, and its long-term effects on health are largely unknown. Using any psychoactive substance carries risks, and Isopropylphenidate should be approached with caution. Harm reduction practices, such as dosage moderation and avoiding interactions with other substances, are essential for minimizing risks.
7. Can Isopropylphenidate cause addiction?
Yes, Isopropylphenidate has the potential to be addictive, especially when used frequently and at high doses. Prolonged use can lead to physical and psychological dependence, making quitting challenging.
8. Are there any known dangerous drug interactions with Isopropylphenidate?
Yes, Isopropylphenidate can interact dangerously with certain substances. Mixing it with alcohol or other stimulants, for example, can increase the risk of adverse effects, including cardiovascular issues and overdose. Always research potential interactions and use caution when combining substances.
9. Can I use Isopropylphenidate for medical purposes?
Isopropylphenidate is not approved for medical use in most countries. It is considered a research chemical and not a prescribed medication. Using it for medical purposes is discouraged and should only be done under the guidance of a healthcare professional.
References
- Markowitz, J. S., Patrick, K. S., Zhu, H., Isopropylphenidate for Treatment of Attention-Deficit/Hyperactivity Disorder and Fatigue-Related Disorders and Conditions
- Markowitz, J. S., Zhu, H.-J., Patrick, K. S. (December 2013). “Isopropylphenidate: An Ester Homolog of Methylphenidate with Sustained and Selective Dopaminergic Activity and Reduced Drug Interaction Liability”. Journal of Child and Adolescent Psychopharmacology. 23 (10): 648–654. doi:10.1089/cap.2013.0074. ISSN 1044-5463.
- Leon, L., Louis, M., Therapeutic lower alkyl esters of alpha-phenyl-alpha-piperidinyl-(2)-acetic acid
- Portoghese, P. S., Malspeis, L. (June 1961). “Relative Hydrolytic Rates of Certain Alkyl (b) dl-α-(2-Piperidyl)-phenylacetates”. Journal of Pharmaceutical Sciences. 50 (6): 494–501. doi:10.1002/jps.2600500611. ISSN 0022-3549.
- Kimko, H. C., Cross, J. T., Abernethy, D. R. (1999). “Pharmacokinetics and Clinical Effectiveness of Methylphenidate:”. Clinical Pharmacokinetics. 37 (6): 457–470. doi:10.2165/00003088-199937060-00002. ISSN 0312-5963.
- Shoptaw, S. J., Kao, U., Ling, W. (January 21 2009). Cochrane Drugs and Alcohol Group, ed. “Treatment for amphetamine psychosis”. Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD003026.pub3. ISSN 1465-1858.
- Hofmann, F. G. (1983). A handbook on drug and alcohol abuse: the biomedical aspects (2nd ed ed.). Oxford University Press. ISBN 9780195030563.
- Talaie, H.; Panahandeh, R.; Fayaznouri, M. R.; Asadi, Z.; Abdollahi, M. (2009). “Dose-independent occurrence of seizure with tramadol”. Journal of Medical Toxicology. 5 (2): 63–67. doi:10.1007/BF03161089. eISSN 1937-6995. ISSN 1556-9039. OCLC 163567183.
- Gillman, P. K. (2005). “Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity”. British Journal of Anaesthesia. 95 (4): 434–441. doi:10.1093/bja/aei210 Freely accessible. eISSN 1471-6771. ISSN 0007-0912. OCLC 01537271. PMID 16051647.
- “Anlage NpSG” (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 24, 2019.
- “Gesetz zur Bekämpfung der Verbreitung neuer psychoaktiver Stoffe” (PDF) (in German). Bundesanzeiger Verlag. Retrieved December 24, 2019.
- “§ 4 NpSG” (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 24, 2019.
- “Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien” (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- “Karar Sayısı : 2016/8548” (PDF) (in Turkish). Resmi Gazete. Retrieved January 15, 2020.
- The Misuse of Drugs Act 1971 (Amendment) Order 2017
- “Controlled Drugs and Substances Act” (in English). Retrieved April 8, 2021.