The market situation for MDA (3,4-methylenedioxyamphetamine) is undergoing significant changes, primarily influenced by shifting regulations and consumer preferences. MDA, once a prominent designer drug and research chemical, is now subject to increased scrutiny and restrictions.
For sellers and vendors, the landscape has become increasingly challenging. Many online platforms have yet to crack down on the sale of designer drugs and research chemicals, making it difficult for sellers to reach their target audience. Some sellers have transitioned to selling similar compounds with fewer legal restrictions, while others have exited the market.
Buyers, on the other hand, need help to source MDA. The dwindling availability of the substance has driven up prices, and individuals interested in purchasing MDA are encountering more barriers than before.
Regulatory changes have played a crucial role in shaping this market situation. Governments worldwide have enacted stricter laws targeting designer drugs, making it harder for vendors to operate and sell MDA openly. This has forced sellers to operate discreetly and has created a more underground market for the substance.
Contents
- 0.1 Summary
- 0.2 History and culture
- 0.3 Chemistry
- 0.4 Pharmacology
- 0.5 Subjective effects
- 0.6 Toxicity
- 0.7 Legal status
- 1 FAQ
- 1.0.1 1. What is MDA?
- 1.0.2 2. How does MDA differ from MDMA (Ecstasy)?
- 1.0.3 3. What are the common street names for MDA?
- 1.0.4 4. Is MDA legal?
- 1.0.5 5. What are the effects of MDA?
- 1.0.6 6. What are the potential risks and side effects of MDA?
- 1.0.7 7. Is MDA addictive?
- 1.0.8 8. Can MDA be used safely?
- 1.0.9 9. Can MDA be used for therapeutic purposes?
- 1.1 References
Summary
3,4-Methylenedioxyamphetamine (MDA), known by its colloquial names “Sally,” “Sass,” or “Sass-a-frass,” belongs to the amphetamine chemical class and is a synthetic entactogen. Its effects encompass long-lasting entactogenic, stimulant, and mild psychedelic qualities. These effects encompass heightened stimulation, anxiety alleviation, increased empathy, affection, sociability, and euphoria upon ingestion.
MDA was first synthesized in 1910, but its psychoactive properties remained undiscovered until 1930. Between 1939 and 1941 and from 1949 to 1957, it was subjected to animal and human trials involving over 500 participants. This research aimed to explore its potential applications as an antidepressant or anorectic. In 1958, it received a patent as a cough suppressant and ataractic, later patented as an anorectic named “Amphedoxamine” in 1961.
Reports suggest that MDA started gaining popularity as a recreational drug around late 1967, preceding its more widely known relative, MDMA (Ecstasy).
Like MDMA, MDA is believed to primarily function as a serotonin-norepinephrine-dopamine reuptake inhibitor and releasing agent. However, with heightened subjective intensity, MDA is notably more potent by weight. It boasts a more extended duration (six to eight hours instead of three to five). It produces traditional serotonergic psychedelic effects like visual distortions, along with increased activity on dopamine, which is thought to contribute to its more significant neurotoxicity.
Presently, the possession of MDA is illegal in most countries, except for limited exceptions related to scientific and medical research.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 4764-17-4 |
|---|---|
| PubChem CID | 1614 |
| DrugBank | DB01509 |
| ChemSpider | 1555 |
| UNII | XJZ28FJ27W |
| ChEMBL | ChEMBL6731 |
| CompTox Dashboard (EPA) | DTXSID40859958 |
| ECHA InfoCard | 100.230.706 |
| Chemical and physical data | |
| Formula | C10H13NO2 |
| Molar mass | 179.219 g·mol−1 |
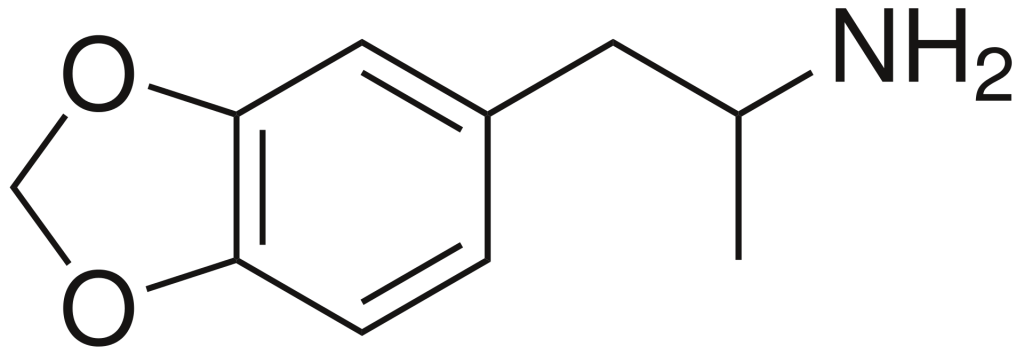
History and culture
MDA was initially synthesized in 1910 by G. Mannish and W. Jacobson. However, its psychoactive properties remained concealed until July 1930, when Gordon Alles conducted self-experiments that unveiled its effects. Subsequently, Alles licensed the compound to Smith, Kline & French. In 1939, the first animal tests were conducted, followed by human trials in 1941 to investigate its potential as a therapy for Parkinson’s disease. Between 1949 and 1957, Smith, Kline & French administered MDA to over 500 human subjects, exploring its use as both an antidepressant and anorectic.
The United States Army also experimented with MDA, code-named EA-1298, to develop a truth serum or incapacitating agent. Tragically, in January 1953, Harold Blauer lost his life after being intravenously injected with 450 mg of the drug.
MDA found its way into patents, becoming a cough suppressant patented by H. D. Brown in 1958, an ataractic patented by Smith, Kline & French in 1960, and an anorectic sold under the trade name “Amphedoxamine” in 1961. Its emergence as a recreational drug occurred around 1967, with a significant seizure of MDA and precursor substances reported in New York in early 1968.
In 1992, a notable batch of pills called “Snowballs” entered mass circulation in Europe, unexpectedly containing MDA instead of MDMA. These pills, dosed inaccurately with nearly 200 mg of the active substance, led to intense hallucinatory experiences across the UK and Europe.
Researchers like Claudio Naranjo and Richard Yensen have also explored MDA’s potential in psychotherapy. In 2010, Matthew Baggott and colleagues conducted a study investigating MDA’s ability to induce mystical experiences and alter vision in healthy volunteers.
Chemistry
MDA, scientifically known as 3,4-methylenedioxyamphetamine, belongs to the amphetamine family of synthetic molecules. Amphetamine-class molecules feature a phenethylamine core characterized by a phenyl ring linked to an amino (NH2) group via an ethyl chain, with an additional methyl substitution at Rα. In the case of MDA, unique substitutions occur at both R3 and R4 positions of the phenyl ring, where oxygen groups are introduced. These oxygen groups are integrated into a methylenedioxy ring through a methylene chain. It’s worth noting that MDA shares this methylenedioxy ring with substances like MDMA, MDAI, and less well-known variants such as MDEA or MMDA.
Pharmacology
MDA functions as both a releasing agent and a reuptake inhibitor for serotonin, dopamine, and norepinephrine neurotransmitters. Additionally, it acts as an agonist for receptors 5-HT2A, 5-HT2B, and 5-HT2C and displays an affinity for TAAR1, α2A, α2B, and α2C adrenergic receptors, as well as 5-HT1A and 5-HT7 receptors.
The impact on serotonin levels may elucidate the shared euphoric and empathogenic effects of MDA and MDMA. Nevertheless, MDA exhibits a higher efficacy in stimulating the 5-HT2A receptor than MDMA. Consequently, MDA tends to induce more psychedelic-like effects, including visual geometric patterns and hallucinations. While MDMA can also generate psychedelic-like visual experiences, they are typically less pronounced than those associated with MDA or necessitate a higher dosage to manifest. It’s worth noting that the precise mechanisms governing these interactions and their contribution to the psychedelic experience remain somewhat elusive.
Subjective effects
MDA, often likened to MDMA, is reported by users to possess heightened stimulant and psychedelic attributes, while its entactogenic effects are typically less intense than those of MDMA. MDA’s effects are also regarded as less predictable, with considerable variation experienced among individuals.
Please note that the following effects are based on anecdotal user reports and the Subjective Effect Index (SEI), so scepticism is advisable.
It’s essential to recognize that these effects may not manifest consistently or reliably, with a higher dosage more likely to evoke the full spectrum of effects. Furthermore, increased doses may escalate the risk of adverse outcomes, including addiction, severe harm, or even fatality ☠.
Physical:
- Stimulation & Sedation: MDA is known to provide both stimulation and sedation paradoxically. While it can impart a “speedy” sensation attributed to its heightened dopamine transporter affinity, it may also induce sedation or a relaxing couch-lock effect, contingent on the user’s mindset and environment. This dual nature can encourage or discourage activities like socializing or dancing, making it popular at festivals and raves.
- Perception of Bodily Heaviness: A sense of bodily weightiness often accompanies sedation induced by this compound.
- Perception of Bodily Lightness: Conversely, feelings of bodily lightness may accompany the stimulation associated with MDA.
- Spontaneous Physical Sensations: MDA’s “body high” is characterized by a euphoric tingling sensation that can range from moderate to overwhelming, reaching its zenith at the peak of the experience.
- Physical Euphoria: The physical euphoria induced by MDA is typically described as profoundly pleasurable and all-encompassing.
- Tactile Enhancement: Users may perceive surfaces such as rugs, carpets, blankets, or skin as softer and more pleasant when under the influence.
- Bodily Control Enhancement
- Vibrating Vision: At higher doses, a rapid, involuntary back-and-forth motion of the eyeballs, known as nystagmus, may blur vision temporarily.
- Temperature Regulation Suppression
- Increased Bodily Temperature: Due to its serotonin-releasing properties, MDA often leads to a substantial and sustained increase in core body and brain temperature. Caution is warranted as excessively high doses can hinder the brain’s ability to regulate internal temperature, potentially leading to serotonin syndrome, a life-threatening condition if left untreated.
- Increased Blood Pressure
- Increased Heart Rate
- Increased Perspiration
- Dehydration: Dry mouth and dehydration are common side effects due to heightened heart rate and metabolism. Users are advised to avoid over-drinking, as water intoxication has occurred in some cases due to excessive consumption.
- Difficulty Urinating: Higher doses may lead to temporary difficulty urinating, attributed to MDA’s promotion of the release of anti-diuretic hormone (ADH). This effect can be alleviated by relaxation or by applying a warm cloth to the genital area to encourage blood flow.
- Appetite Suppression
- Pupil Dilation
- Excessive Yawning
- Stamina Enhancement
- Teeth Grinding: This is typically more pronounced at moderate to higher doses and resembles the teeth grinding associated with MDMA.
- Temporary Erectile Dysfunction
- Seizure: Although rare, seizures can occur in individuals predisposed to them, especially at higher-than-recommended doses or in physically demanding conditions, such as dehydration, fatigue, or malnutrition.
Visual:
- MDA’s visual effects are less consistent and selective compared to traditional psychedelics. They are more likely to occur with chemically pure, high-dose MDA experiences, often toward the end of the experience or when combined with cannabis. These effects can manifest even in users with no prior psychedelic experience.
- Enhancements: MDA introduces mild visual enhancements, including improved visual acuity, colour enhancement, and pattern recognition enhancement.
- Distortions: Visual distortions such as tracers, after-images, and symmetrical texture repetition can be observed.
- Geometry: Visual geometry under MDA is more akin to psilocin than LSD. It’s described as intricate, abstract, organic, structured, dimly lit, monotone in colour (primarily blues and greys), glossy in shading, sharp in edges, small in size, fast in speed, smooth in motion, with both round and angular corners. Higher doses are more likely to induce level 8A visual geometry over level 8B.
- Hallucinatory States: MDA can produce low and high-level hallucinatory states at high doses, although their occurrence is less consistent and reproducible than other psychedelics. These effects often emerge during the experience’s offset and may include peripheral information misinterpretation, transformations, external hallucinations (autonomous entities, settings, sceneries, landscapes, perspective hallucinations, scenarios, and plots), and internal hallucinations. The latter, experienced at extremely high doses, often involve hypnagogic scenarios resembling memory replays from the preceding hours.
Cognitive:
- MDA’s cognitive effects intensify with dosage, including moderate to extreme mental stimulation, feelings of love, openness, empathy, and powerful euphoria. They encompass psychedelic, entactogenic, and stimulant cognitive effects, with prominent features such as anxiety suppression, cognitive euphoria, enhanced empathy, affection, and sociability, emotion enhancement, time distortion, disinhibition, increased music appreciation, heightened sense of humour, laughter fits, compulsive redosing, novelty enhancement, creativity enhancement, focus enhancement (more effective at lower to moderate doses), immersion enhancement, motivation enhancement, increased libido, mindfulness, thought acceleration, and wakefulness. Users often report feeling heavily sedated or “floored” compared to typical stimulants.
Auditory:
- Auditory effects include enhancements, hallucinations, and distortions.
Transpersonal:
- Transpersonal effects involve existential self-realization and feelings of unity and interconnectedness. These effects can be more pronounced in large crowds at raves and musical events.
After:
- The comedown or “offset” phase of MDA can be uncomfortable and harmful due to neurotransmitter depletion. This phase may include anxiety, appetite suppression, brain zaps, cognitive fatigue, depression, derealization, dream suppression or potentiation, sleep paralysis, irritability, motivation suppression, thought deceleration, thought disorganization, and wakefulness.
Toxicity
Based on anecdotal evidence from individuals in the Psychonaut community who have experimented with MDA, trying the drug by itself at low to moderate doses and using it sparingly is not associated with immediate adverse health effects. However, absolute safety cannot be guaranteed. It is crucial to emphasize the importance of conducting independent research to ensure the safety of any substance, especially when considering combinations of two or more substances.
It’s worth noting that Harold Blauer tragically passed away in January 1953 after receiving an intravenous injection of 450 mg of MDA.
Comparatively, MDA is recognized to be more neurotoxic when compared to substances like MDMA or MDE.
Using harm reduction practices is strongly recommended when using this substance.
Tolerance and Addiction Potential
Like other stimulants, chronic MDA use carries a moderate risk of addiction and a high potential for abuse, potentially leading to psychological dependence in specific individuals. Those who develop addiction may experience cravings and withdrawal symptoms when discontinuing use.
Tolerance to MDA’s psychedelic effects typically builds quickly after ingestion. However, tolerance to its stimulant and entactogenic effects develops more slowly with repeated and heavy use, with variations among individuals. It takes approximately 3 days for the tolerance to decrease by half and 7 days to return to baseline (assuming no further consumption). MDA induces cross-tolerance with all psychedelics and some stimulants, meaning that consuming MDA can reduce the effectiveness of other psychedelics and certain stimulants.
Dangerous Interactions
- 25x-NBOMe & 25x-NBOH: Combining these compounds with MDA should be strictly avoided due to the risk of excessive stimulation and heart strain, potentially leading to increased blood pressure, vasoconstriction, panic attacks, thought loops, seizures, and, in extreme cases, heart failure.
- Alcohol: Combining alcohol with stimulants can be dangerous as it can lead to accidental over-intoxication. Stimulants mask the depressant effects of alcohol, making it difficult to assess one’s level of intoxication. When the stimulant’s effects wear off, the depressant effects can emerge unopposed, potentially resulting in blackouts and severe respiratory depression. If combining these substances, users should strictly limit their alcohol intake per hour.
- DXM: Combining MDA with DXM should be avoided due to DXM’s inhibitory effects on serotonin and norepinephrine reuptake. This combination increases the risk of panic attacks and hypertensive crisis, or serotonin syndrome, when combined with serotonin releasers like MDMA.
- MDMA: Combining MDA with MDMA or other stimulants may amplify any neurotoxic effects and risk excessive blood pressure and heart strain (cardiotoxicity).
- MXE: Reports suggest that combining MDA with MXE can dangerously elevate blood pressure and increase the risk of mania and psychosis.
- Dissociatives: Both MDA and dissociatives carry the risk of inducing delusions, mania, and psychosis, and this risk may increase when the two are combined.
- Stimulants: Combining MDA with other stimulants like cocaine can significantly raise heart rate and blood pressure to dangerous levels.
- Tramadol: Tramadol is known to lower the seizure threshold, and combining it with stimulants may further increase this risk.
- Cocaine: Combining MDA with cocaine can place additional strain on the heart.
- Amphetamine: This combination may result in suicidal mania, paranoia, and hallucinations approximately five days after discontinuing long-term amphetamine usage, potentially due to neural network disequilibrium related to MDA’s lack of calcium channel action.
Serotonin Syndrome Risk
Combining MDA with the following substances can lead to dangerously elevated serotonin levels, potentially causing serotonin syndrome, which requires immediate medical attention and can be fatal if left untreated:
- MAOIs: Such as banisteriopsis caapi, Syrian rue, phenelzine, selegiline, and moclobemide.
- Serotonin Releasers: MDMA, 4-FA, methamphetamine, methylone, and αMT.
- SSRIs: Such as citalopram and sertraline.
- SNRIs: Such as tramadol and venlafaxine.
- 5-HTP.
Legal status
Internationally, MDA is classified as a Schedule I substance under the Convention on Psychotropic Substances 1971.
Here is the legal status of MDA in various countries:
- Australia: MDA is considered a controlled substance.
- Austria: MDA possession, production, and sale are illegal under the SMG (Suchtmittelgesetz Österreich).
- Canada: MDA is listed as a Schedule I substance in the CSDA.
- Germany: MDA has been controlled under Anlage I BtMG (Narcotics Act, Schedule I) since September 1, 1984. Manufacturing, possessing, importing, exporting, buying, selling, procuring, or dispensing it without a license is illegal.
- Russia: MDA is classified as a Schedule I prohibited substance.
- Switzerland: MDA is recognized as a controlled substance, named explicitly under Verzeichnis D.
- The Netherlands: MDA possession, production, and sale are illegal in the Netherlands.
- United Kingdom: MDA is classified as a class A drug.[citation needed]
- United States: MDA is categorized as a Schedule I drug.
FAQ
1. What is MDA?
MDA, also known as 3,4-methylenedioxyamphetamine, is a synthetic substance in the amphetamine class of chemicals. It is recognized for its entactogenic, stimulant, and mild psychedelic effects, often including enhanced empathy, sociability, stimulation, and mild hallucinations.
2. How does MDA differ from MDMA (Ecstasy)?
MDA and MDMA (Ecstasy) are related substances, but they have some differences. MDA is generally considered to have more pronounced stimulant and psychedelic qualities compared to MDMA. While both substances can enhance empathy and sociability, MDA’s effects are often more stimulating and hallucinogenic.
3. What are the common street names for MDA?
MDA is known by various street names, including “Sass,” “Sass-a-frass,” “Sally,” and others. These names may vary depending on the region and local slang.
4. Is MDA legal?
The legal status of MDA varies by country and region. In many places, it is classified as a controlled substance and is illegal to manufacture, possess, distribute, or use without a prescription or specific license. It is essential to be aware of the legal status of MDA in your area before considering its use.
5. What are the effects of MDA?
MDA produces enhanced sociability, empathy, stimulation, mild psychedelic visuals, and increased energy. Users may also experience euphoria, increased tactile sensations, and altered perception. However, individual responses can vary.
6. What are the potential risks and side effects of MDA?
The use of MDA is associated with several potential risks, including increased heart rate, elevated blood pressure, dehydration, difficulty urinating, and temperature regulation issues. More severe side effects, such as hallucinations, anxiety, and, in extreme cases, seizures or serotonin syndrome, can occur.
7. Is MDA addictive?
MDA has the potential for psychological dependence and addiction, especially with chronic use. Users may experience cravings and withdrawal symptoms if they suddenly stop using the substance. It is crucial to use MDA sparingly and be aware of the risks associated with its use.
8. Can MDA be used safely?
The safety of using MDA depends on various factors, including dosage, frequency of use, and an individual’s health and mental state. Harm reduction practices, such as accurate dosing, staying hydrated, and avoiding risky combinations with other substances, can help minimize potential harm.
9. Can MDA be used for therapeutic purposes?
Some researchers have explored the potential therapeutic uses of MDA, primarily in psychotherapy. However, its legal status and potential risks make it a challenging substance to use for therapeutic purposes.
References
- Mannich, C., Jacobsohn, W., Mannich, C. (January 1910). “Über Oxyphenyl‐alkylamine und Dioxyphenyl‐alkylamine”. Berichte der deutschen chemischen Gesellschaft. 43 (1): 189–197. doi:10.1002/cber.19100430126. ISSN 0365-9496.
- Schoenfeld, Eugene. “Hippocrates”. Berkeley Barb November 24-30, 1967: 7 (Independent Voices) | http://voices.revealdigital.com/cgi-bin/independentvoices?a=d&d=BFBJFGD19671124.1.7
- $700,000 Awarded to Estate of Army Drug Test Victim, 1987
- “Methylenedioxy Amphetamine (MDA).” Microgram. Bureau of Drug Abuse Control. Feb 1968. 1(5):4-5 (Erowid.org) | https://erowid.org/library/periodicals/microgram/microgram_1968_02_v01n05.pdf
- Power, Mike (2 May 2013). Drugs 2.0: The Web Revolution That’s Changing How the World Gets High. Portobello Books Ltd. ISBN 9781846274596.
- Naranjo, C., Shulgin, A. T., Sargent, T. (1967). “Evaluation of 3,4-Methylenedioxyamphetamine (MDA) as an Adjunct to Psychotherapy”. Pharmacology. 17 (4): 359–364. doi:10.1159/000137100. ISSN 0031-7012.
- Yensen, R., Di Leo, F. B., Rhead, J. C., Richards, W. A., Soskin, R. A., Turek, B., Kurland, A. A. (October 1976). “MDA-assisted psychotherapy with neurotic outpatients: a pilot study”. The Journal of Nervous and Mental Disease. 163 (4): 233–245. doi:10.1097/00005053-197610000-00002. ISSN 0022-3018.
- Baggott, M. J., Siegrist, J. D., Galloway, G. P., Robertson, L. C., Coyle, J. R., Mendelson, J. E. (2 December 2010). “Investigating the Mechanisms of Hallucinogen-Induced Visions Using 3,4-Methylenedioxyamphetamine (MDA): A Randomized Controlled Trial in Humans”. PLOS ONE. 5 (12): e14074. doi:10.1371/journal.pone.0014074. ISSN 1932-6203.
- Lewin, A. H., Miller, G. M., Gilmour, B. (1 December 2011). “Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class”. Bioorganic & Medicinal Chemistry. 19 (23): 7044–7048. doi:10.1016/j.bmc.2011.10.007. ISSN 1464-3391.
- Wallach, J. V. (January 2009). “Endogenous hallucinogens as ligands of the trace amine receptors: a possible role in sensory perception”. Medical Hypotheses. 72 (1): 91–94. doi:10.1016/j.mehy.2008.07.052. ISSN 0306-9877.
- Giovanni, G. D., Matteo, V. D., Esposito, E. (11 November 2008). Serotonin-Dopamine Interaction: Experimental Evidence and Therapeutic Relevance. Elsevier. ISBN 9780444532350.
- Rothman, R. B., Baumann, M. H. (May 2009). “Serotonergic drugs and valvular heart disease”. Expert Opinion on Drug Safety. 8 (3): 317–329. doi:10.1517/14740330902931524. ISSN 1744-764X.
- Nash, J. F., Roth, B. L., Brodkin, J. D., Nichols, D. E., Gudelsky, G. A. (15 August 1994). “Effect of the R(-) and S(+) isomers of MDA and MDMA on phosphatidyl inositol turnover in cultured cells expressing 5-HT2A or 5-HT2C receptors”. Neuroscience Letters. 177 (1–2): 111–115. doi:10.1016/0304-3940(94)90057-4. ISSN 0304-3940.
- Ray, T. S. (2 February 2010). “Psychedelics and the human receptorome”. PloS One. 5 (2): e9019. doi:10.1371/journal.pone.0009019. ISSN 1932-6203.
- Johnson, M. P., Hoffman, A. J., Nichols, D. E. (December 1986). “Effects of enantiomers of MDA, MDMA and related analogues on [3H]serotonin and [3H]dopamine release from superfused rat brain slices”. European Journal of Pharmacology. 132 (2–3): 269–276. doi:10.1016/0014-2999(86)90615-1. ISSN 0014-2999.
- The History Channel documented details of his death here http://www.youtube.com/watch?v=ySw-0uY4CUA See minute 2:38 onward.
- http://www.drogen-info-berlin.de/htm/mda.html
- Talaie, H.; Panahandeh, R.; Fayaznouri, M. R.; Asadi, Z.; Abdollahi, M. (2009). “Dose-independent occurrence of seizure with tramadol”. Journal of Medical Toxicology. 5 (2): 63–67. doi:10.1007/BF03161089. eISSN 1937-6995. ISSN 1556-9039. OCLC 163567183.
- Gillman, P. K. (2005). “Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity”. British Journal of Anaesthesia. 95 (4): 434–441. doi:10.1093/bja/aei210 Freely accessible. eISSN 1471-6771. ISSN 0007-0912. OCLC 01537271. PMID 16051647.
- “CONVENTION ON PSYCHOTROPIC SUBSTANCES 1971” (PDF). United Nations. Retrieved December 15, 2019.
- “Criminal Code Regulations 2019”. Office of Parliamentary Counsel. Retrieved December 15, 2019.
- “Controlled Drugs and Substances Act – SCHEDULE I”. Government of Canada. Retrieved December 15, 2019.
- “Erste Verordnung zur Änderung betäubungsmittelrechtlicher Vorschriften” (in German). Bundesanzeiger Verlag. Retrieved December 15, 2019.
- “Anlage I BtMG” (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 15, 2019.
- “§ 29 BtMG” (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 15, 2019.
- Resolution of the Government of the Russian Federation
- “Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien” (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- Koninkrijksrelaties, M. van B. Z. en, Opiumwet
- Passie, T., Benzenhöfer, U. (June 2016). “The History of MDMA as an Underground Drug in the United States, 1960-1979”. Journal of Psychoactive Drugs. 48 (2): 67–75. doi:10.1080/02791072.2015.1128580. ISSN 0279-1072.